Synthesis of technomimetic molecules: towards rotation control in single-molecular machines and motors
Gwénaël
Rapenne
*
NanoSciences Group, CEMES-CNRS, 29 rue Jeanne Marvig, BP 94347, F-31055 Toulouse Cedex 4, France. E-mail: rapenne@cemes.fr; Fax: (+33) 562 25 79 99; Tel: (+33) 562 25 78 41
First published on 24th February 2005
Abstract
Technomimetic molecules are molecules designed to imitate macroscopic objects at the molecular level, also transposing the motions that these objects are able to undergo. This article focuses on technomimetic molecules with rotary motions, including gears, wheelbarrows and motors. Following the bottom-up approach the synthesis of technomimetic molecules grants access to the study of mechanical properties at the molecular level. These molecules are designed to operate as single molecules on surfaces under the control of the tip of a scanning tunneling microscope or atomic force microscope.
 Gwénaël Rapenne | Gwénaël Rapenne was born in Belfort (France) and graduated in chemistry from Louis Pasteur University of Strasbourg in 1995 with honors. After a short stay in Cambridge (UK) working as an ERASMUS student in the group of Dr Martin J. Mays on Group VI dinuclear organometallic complexes, he started a PhD thesis in Strasbourg under the supervision of Dr Jean-Pierre Sauvage and Dr Christiane Dietrich-Buchecker working on the synthesis and the resolution of molecular knots. Gwénaël received his PhD in 1998 and then spent one year as a Lavoisier postdoctoral fellow working on Fullerenes with Professor François Diederich at ETH Zürich (Switzerland). In 1999, he joined the NanoSciences Group at CEMES (CNRS) as a Maître de Conférences at the University Paul Sabatier of Toulouse to work in the field of single-molecular machines and motors. |
Introduction
In the macroscopic world, the movement of rotation is at the source of many examples of machines and motors. The discovery of the wheel in ancient times has been at the origin of considerable developments of our civilization and the exploitation of the rotary movement resulted in the design of motors which allowed the industrial revolution. Today, rotary motors are very common in all the objects we use daily, such as cars, kitchen robots, clocks and many tools. At the microscopic level, Mother Nature developed a fascinating machinery called ATP synthase,1 based on the use of the rotation movement. In the continuous miniaturization of electronic and mechanic devices, molecules are expected to play a major role since multistep chemical synthesis allows chemists to prepare tailor-made molecules with predetermined shape and programmed movement. In the bottom-up strategy, artificial molecular machines and motors have recently emerged as a new field of chemistry related to a newly explored dimension of molecular sciences: controlled movement at the molecular scale.2 The ultimate miniaturization of electronic and mechanic devices is reached when addressing one single molecule and not a population of molecules in solution. Therefore, operating at a molecular scale is less important than working at a single-molecular level. If individual electronic or mechanic components can reach the nanometer scale, the door will be open to real technological applications.Recent advances in the imaging and manipulation of single molecules3 has stimulated much interest in the synthesis of molecules exhibiting unique electronic properties but also very special mechanical properties. For that purpose, we designed technomimetic molecules to transpose macroscopic objects at the molecular level, including the motions that these objects are able to undergo. This article focuses on technomimetic molecules with rotary motions such as gears, wheelbarrows and motors. Let us start with the presentation of what Mother Nature is able to do, which is a major source of inspiration for the chemists' community.
ATP synthase, a biological rotary motor
Each man consumes an average of 40 kg of ATP per day. Fortunately, the ADP formed during the hydrolysis of ATP is recycled. The machinery where this process takes place is ATP synthase,1 an enzyme present in the cell membrane. The energy necessary for the cell is produced by the conversion of ATP into ADP and phosphate. The stock of ATP can be reconstituted by the reverse mechanism. It transforms into chemical energy the electrical energy due to the trans-membrane proton concentration gradient.As shown in Fig. 1, ATP synthase is constituted of two parts: the first one, called F0, is incorporated in the membrane of the cells; the second, called F1, is in the extracellular medium. F0 is composed of a mobile part, the rotor, constituted of 10–12 proteic subunits (c) and of a stationary part, the stator (b) which is partly outside of the membrane. In this region, when a proton is fixed on a negatively charged subunit c, the subunit is neutralized and subsequently moves towards a less polar environment, i.e. the membrane, leading away the whole rotor. The rotor, which is a proton-fuelled turbine, extends into a tree (γ), the movement of which activates in turn the three αβ subunits of the F1 part, synthesizing ATP.
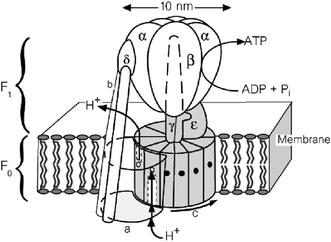 | ||
| Fig. 1 ATP synthase incorporated in a membrane. | ||
It is extraordinary to note that this device is rotating in one direction to produce ATP and in the opposite direction to produce energy, which consumes ATP. Inspired by this high-performance natural macromolecular system, different groups of chemists challenged themselves to reach such a Holy Grail, although smaller in size, projecting to build molecular motors.4 Indeed, this natural machinery is constituted by an assembly of different biological macromolecules with a size of 10 nm in diameter and of about 25 nm in length, which is much larger than the usual molecular size.
The movement of rotation at a single-molecular scale
Some very elegant examples of molecular rotary motors have been described by Feringa, Kelly, Leigh and others,4 but the molecular motors proposed and synthesized so far have been studied in solution and have many degrees of freedom. Their behaviour is the average behaviour of a weak assembly of molecules and not of a single molecule. By contrast, the molecules described in this article have been designed with the intention to study and manipulate them individually with the tip of a Scanning Tunneling Microscope (STM). This clearly implies that the molecules be particularly rigid and have minimal degrees of freedom in order to be manipulated on a surface with a maximum control on their movement.A non-directional monomolecular rotor
The first real molecular rotor to be studied was hexa-tert-butyldecacyclene, represented in Fig. 2.5 It is constituted of a polyaromatic platform of 1.5 nm diameter built on six tert-butyl legs, these saturated hydrocarbon groups allowing the isolation of the board from the metallic surface used in the study. The STM allowed not only the imaging of the molecule but also, once the position of the molecule was known precisely, to manipulate it. On the left of Fig. 2 is shown a monolayer of this molecule deposited on a copper surface (Cu 100).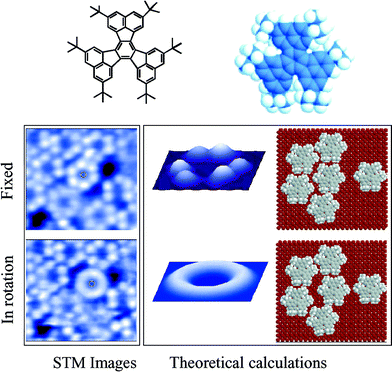 | ||
| Fig. 2 The upper part shows two chemical representations of hexa-tert-butyldecacyclene. STM images of a monolayer (left); and images calculated by the ESQC method (centre) showing only the molecule located in the middle part of the monolayer. This molecule is fixed (top) and in rotation (bottom). On the right are shown two calculated representations of the arrangement of the adsorbed monolayer of molecules. | ||
The molecule of interest is located in the middle part of the image. Moving this molecule with the tip of the STM slightly to the right towards the black zone corresponding to a hole in the monolayer induces a change in the image. After its translation, the molecule, initially fixed on the surface by incarceration between its neighbours, started to rotate. The white spots, corresponding to the legs of motionless molecules, are not localized any more and the molecule in the centre of the bottom-left image is like a torus, indicating a rotation of the whole molecule. This molecule is really acting as a monomolecular rotor but its rotation is controlled by its environment. Without this supramolecular network, the molecule loses this property, since it needs its neighbours in a supramolecular bearing to act as a rotor. Moreover, this rotation is not directional but is a random, thermally activated rotation.
A single-molecular motor: towards the control of the rotation
A motor is a machine which consumes energy to create work continuously via a unidirectional and controlled movement. The major difficulties concern the control of the directionality of the rotation, the continuous response to the stimulus and the manipulation of a single molecule. For instance, the molecule shown in the previous paragraph does not undergo a directional rotation, its movement can be seen as random oscillations. Our current research project consists of the design and synthesis of a rotor able to act, as such, independently of its environment and with a control on the directionality of the rotation. In our strategy,6 the source of energy will be electric and our target molecule is supposed to convert an electric current into a directionally-controlled rotary motion. The motor we would like to present here has been designed to be individually addressed after its deposition between two nanoelectrodes separated by a few nanometers (i.e. a nanojunction).The active part of our molecular motor is represented in Fig. 3. Its comprises a stator, i.e. one part fixed between two electrodes, and on this stator is connected a rotor which should transform a current of electrons into a unidirectional rotational motion. The rotor is a rigid aromatic platform constructed around a cyclopentadienyl ligand with five linear and rigid arms, each terminated by an electroactive group. As the electroactive group, ferrocene was selected because it exhibits reversible oxidation in various solvents.7 The stator is a hydrotris(indazolyl)borate ligand of the family of scorpionates developed by Trofimenko8 with a piano stool shape. The joint between the rotor and the stator is a ruthenium(II) ion to obtain a stable molecule bearing zero net charge, both criteria being essential for surface deposition and hence for building single-molecule nanomotors. The upper part should be free to turn whilst the basis should stay still, anchored on the surface between the two electrodes of the addressing system.
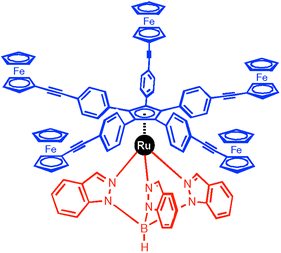 | ||
| Fig. 3 Structure of the active part of a single-molecular motor. The lower ligand is the stator (in red) and the upper ligand is the rotor with five ferrocene-terminated arms (in blue). The ruthenium plays the role of joint between the two ligands. | ||
This molecule was synthesized starting from pentaphenyl cyclopentadiene which was brominated selectively in the para positions and at the saturated carbon of the cyclopentadiene ring.9 This new ligand was coordinated to ruthenium by reaction with Ru3(CO)12 and therefore the tris(indazolyl)borate ligand was introduced before the last step, which involved the coupling of five ethynyl ferrocene electroactive groups. We also developed the synthesis of a tris(indazolyl)borate ligand incorporating some anchoring groups10 in view of its deposition on an isolating surface to be studied on the molecular scale with an Atomic Force Microscope (AFM).
The concept of our electron-fuelled molecular rotary motor is shown in Fig. 4. The electroactive group (EG) closest to the anode would be oxidized (oxidized form EG+) and pushed back by electrostatic repulsion as it has been shown for a [60]-fullerene between two electrodes.11 This motion corresponds to a fifth of a turn. As a result, the oxidized electroactive group would approach the cathode and subsequently be reduced. At the same time, a second electroactive group would come close to the anode and a second cycle would occur. A complete 360° turn would be achieved after five cycles, corresponding to the transport of five electrons from the cathode to the anode. This would correspond to the conversion of an electron flow into a movement of rotation, i.e. a redox-triggered molecular rotary motor. In order for the rotation to be directional, the molecule should be placed in a dissymmetrical environment. This could be achieved either by its disposition in the nanojunction, or by a secondary electric field perpendicular to the nanojunction.
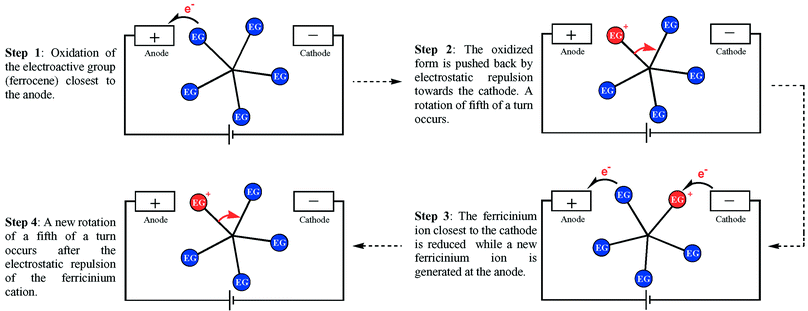 | ||
| Fig. 4 Schematic representation of a molecule placed between the two electrodes of a nanojunction (EG stands for electroactive group). The transfer of electrons from the cathode to the anode through successive oxidation and reduction processes is expected to result in the clockwise rotation of the entire upper part of the molecule. On this figure is represented a fifth of a turn corresponding to the movement induced by the transfer of one electron. | ||
Once the molecule was in our hands, we checked all the requirements for such a molecule to operate as a molecular motor: (i) the oxidation potential of the iron is lower compared to the ruthenium centre which is compatible with our objective, in the sense that the ruthenium centre will remain inert towards the redox cycles of the peripheral electroactive groups; (ii) electrochemical processes are reversible, showing the robustness of the molecule towards oxidation; (iii) no intervalence band was observed showing that the electronic communication between two iron centres is null or very weak – electronic communication is an unwanted phenomenon here since it would allow a charge transport by intramolecular electron hopping between different ferrocene centres, without real motion of the rotor; and (iv) the barrier of rotation of the rotor is very low, as shown both by NMR and by DFT calculations.
The deposition of the complex functionalized with anchoring groups and the observation of the conversion of an electric current into a controlled rotary movement remains to be attempted, using suitable methods such as analysis of the time dependence of the current and scanning probe microscopes.
An organometallic molecular turnstile with a correlated gear effect
During the synthesis of the active part of our molecular motor, we have been particularly interested by the molecule represented in Fig. 5. This molecule was an intermediate in our synthetic pathway and was an interesting candidate to study the random rotation of the upper part of the molecule for which an interesting behaviour was brought to the fore.9 Owing to the steric hindrance imposed by the three parts of the lower tripodal ligand, the rotation of the upper cyclopentadienyl ligand (random rotation induced by the thermal bath surrounding them) results in the rotation of the para-bromophenyl paddles. Since the X-ray structure showed the two ligands to be interpenetrating and since the 1H-NMR showed the five p-bromophenyl rings to be equivalent, the rotation of the Cp ring should only be possible if the p-bromophenyl groups tipped over to settle in the vacant spaces of the tripodal ligand like a Fosbury jump.12 In Fig. 5 is represented the secondary rotation of the p-bromophenyl rings (action 2) induced by the rotation of the upper Cp ligand (action 1). These two rotations are correlated and this molecule behaves like a molecular turnstile with a correlated gear effect.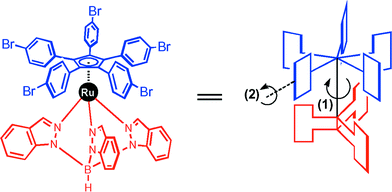 | ||
| Fig. 5 Rotation of the upper Cp ligand (action 1) results in the paddles tipping over (action 2). | ||
A molecular wheelbarrow
In the field of single molecule manipulation it is a challenge to manipulate a molecule which would at the same time undergo translation and rotation motions.13 In the case of a macroscopic wheelbarrow, pushing the wheelbarrow results in the rotation of the wheel. In Fig. 6 is shown a molecular analogue of a wheelbarrow. Its skeleton is made of Polycyclic Aromatic Hydrocarbons (PAHs)14 which, due to their rigidity, are easily manipulated by the STM tip. The wheelbarrow is constituted of two legs (3,5-di-tert-butyl phenyl groups, in green) and two wheels (ethynyl triptycene groups, in red) connected to a polycyclic aromatic hydrocarbon platform. It corresponds to a dissymmetrized lander from the family of molecules developed specifically for their study on various metallic surfaces.15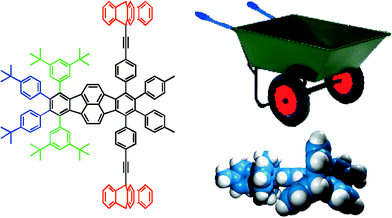 | ||
| Fig. 6 Chemical structure of a molecular wheelbarrow (left), side view of the CPK model showing the minimum energy conformation of the molecular analogue (bottom right), and its macroscopic analogue (top right). | ||
The two 3,5-di-tert-butyl phenyl legs (in green) which equip the left side of our molecule were shown to be held in a conformation in which the phenyl groups are nearly perpendicular to the main aromatic board. Moreover, tert-butyl groups connected to PAHs are also used to increase the organic solubility and are easily observed by STM techniques, inducing a good contrast in the image.16 The two 4-tert-butyl phenyl groups (in blue) play the role of handles for subsequent manipulation with the tip of the microscope. The right-hand side corresponds to the axle with two 9-triptycenyl groups of C3 symmetry acting as wheels (in red). We opted for two wheels instead of one for obvious synthetic reasons. Fig. 6 shows one of the possible conformations of this molecule obtained by semiempirical calculation with the two three-cogged wheels which can freely rotate around the axle due to the acetylenic spacers.
The synthesis of a polyaromatic hydrocarbon designed by analogy with a wheelbarrow has been achieved in 12 steps and an overall yield of 2%.17 After deposition, the molecules have been imaged by STM on Cu(100) terraces as shown in Fig. 7 and identified by comparison with calculated images.18
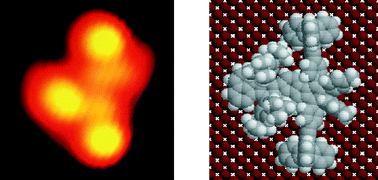 | ||
| Fig. 7 Experimental STM image of the molecular wheelbarrow on Cu(100) (left) and the molecular conformation corresponding to the calculated image (right). | ||
Unfortunately, we have not yet been able to obtain an understandable movement of the molecule since it interacts very strongly with the metallic surface despite the 3,5-di-tert-butyl phenyl legs. We are hoping to reproduce the mechanical behaviour of a wheelbarrow at the molecular level, i.e. to convert the translation movement of the tip into the rotation of the wheels by the use of alternative surfaces, for instance insulating surfaces such as NaCl on copper. It must be noted that if such rotation of the wheels occurs, it should be directional. ‘Seeing’ the rotation of a wheel on a surface is a major goal which meets our main target to build a single-molecular motor.
Future outlook
With the study of controlled rotation on single molecules, we are on the way to a molecular motor able to be manipulated and studied at the single-molecular level. One of the big issues is to have sufficiently rigid systems in order to have exploitable movements inducing a mechanical work. Although some progress has been made in the field of molecular machines and motors, it is still a long way to practical applications.Acknowledgements
Professor Jean-Pierre Launay and Dr Christian Joachim are gratefully acknowledged for having welcomed me into their group and for allowing me to develop the research topics I wish. I also would like to thank Dr Jean-Pierre Sauvage and Dr Christiane Dietrich-Buchecker for being constant sources of inspiration. Dr Alexandre Carella is especially acknowledged. He was the first PhD student to join me on this adventure. Dr Gorka Jimenez-Bueno, Dr Leonhard Grill and Dr Francesca Moresco are also thanked for their contribution to this work. I am also grateful to the European Union, CNRS and Université Paul Sabatier for financial supports. Dr Isabelle M. Dixon is warmly acknowledged for her comments on this manuscript.References
- J. F. Walker, Angew. Chem., Int. Ed., 1998, 37, 2308–2319 CrossRef.
- Special Issue on Molecular Machines: see Acc. Chem. Res., 2001, 34, pp. 409–522 Search PubMed.
- F. Moresco, Phys. Rep., 2004, 399, 175–225 CrossRef CAS and refs. cited therein.
- T. R. Kelly, H. De Silva and R. A. Silva, Nature, 1999, 401, 150–152 CrossRef CAS; T. R. Kelly, R. A. Silva, H. De Silva, S. Jasmin and Y. Zhao, J. Am. Chem. Soc., 2000, 122, 6935–6949 CrossRef CAS; N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152–155 CrossRef CAS; M. K. J. ter Wiel, R. A. van Delden, A. Meetsma and B. L. Feringa, J. Am. Chem. Soc., 2003, 125, 15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076–15
076–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 CrossRef CAS; K. Tashiro, K. Konishi and T. Aida, J. Am. Chem. Soc., 2000, 122, 7921–7926 CrossRef CAS; M. Ikeda, M. Takeuchi, S. Shinkai, F. Tani, Y. Naruta, S. Sakamoto and K. Yamaguchi, Chem. Eur. J., 2002, 8, 5541–5550 CrossRef CAS; D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Nature, 2003, 424, 174–179 CrossRef CAS; J. V. Hernandez, E. R. Kay and D. A. Leigh, Science, 2004, 306, 1532–1537 CrossRef CAS; D. Horinek and J. Michl, J. Am. Chem. Soc., 2003, 125, 11 900–11 910 CrossRef CAS; H. Jian and J. M. Tour, J. Org. Chem., 2003, 68, 5091–5103 CrossRef CAS.
086 CrossRef CAS; K. Tashiro, K. Konishi and T. Aida, J. Am. Chem. Soc., 2000, 122, 7921–7926 CrossRef CAS; M. Ikeda, M. Takeuchi, S. Shinkai, F. Tani, Y. Naruta, S. Sakamoto and K. Yamaguchi, Chem. Eur. J., 2002, 8, 5541–5550 CrossRef CAS; D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Nature, 2003, 424, 174–179 CrossRef CAS; J. V. Hernandez, E. R. Kay and D. A. Leigh, Science, 2004, 306, 1532–1537 CrossRef CAS; D. Horinek and J. Michl, J. Am. Chem. Soc., 2003, 125, 11 900–11 910 CrossRef CAS; H. Jian and J. M. Tour, J. Org. Chem., 2003, 68, 5091–5103 CrossRef CAS. - J. K. Gimzewski, C. Joachim, R. Schlitter, V. Langlais and H. Tang, Science, 1998, 281, 531–533 CrossRef CAS.
- A. Carella, G. Rapenne and J.-P. Launay, New J. Chem., 2005, 29, 288–290 RSC.
- D. Astruc, Acc. Chem. Res., 2000, 33, 287–298 CrossRef CAS.
- S. Trofimenko, Scorpionates: The Coordination Chemistry of Polypyrazolylborate Ligands, Imperial College Press, London, 1999 Search PubMed.
- A. Carella, J. Jaud, G. Rapenne and J.-P. Launay, Chem. Commun., 2003, 2434–2435 RSC.
- A. Carella, T. Cox, G. Vives, J. Jaud, G. Rapenne and J.-P. Launay, manuscript in preparation.
- H. Park, J. Park, A. K. L. Lim, E. H. Anderson, A. P. Alivisatos and P. L. McEuen, Nature, 2000, 407, 57–60 CrossRef.
- From the name of Dick Fosbury who revolutionized high jump in 1968, by introducing the technique of leaping backward over the bar, and rolling around it.
- C. Joachim, H. Tang, F. Moresco, G. Rapenne and G. Meyer, Nanotechnology, 2002, 13, 330–335 CrossRef CAS.
- A. J. Berresheim, M. Müller and K. Müllen, Chem. Rev., 1999, 99, 1747–1786 CrossRef CAS.
- A. Gourdon, Eur. J. Org. Chem., 1998, 2797–2801 CrossRef CAS.
- F. Moresco, G. Meyer, K.-H. Rieder, H. Tang, A. Gourdon and C. Joachim, Phys. Rev. Lett., 2001, 86, 672–675 CrossRef CAS.
- G. Jimenez-Bueno and G. Rapenne, Tetrahedron Lett., 2003, 44, 6261–6263 CrossRef.
- L. Grill, F. Moresco, K.-H. Rieder, G. Jimenez-Bueno, C. Wang, G. Rapenne and C. Joachim, Surf. Sci., submitted for publication Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |
