Construction of biotinylated peptide nanotubes for arranging proteins†
Sachiko
Matsumura‡
a,
Shinobu
Uemura§
a and
Hisakazu
Mihara
*b
aCorporate Research Laboratory, Corporate Research Group, Fuji Xerox Co. Ltd., 430 Sakai, Nakai-machi, Ashigarakami-gun, Kanagawa 259-0157, Japan
bDepartment of Bioengineering, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, 4259-B-40 Nagatsuta-cho, Midori-ku, Yokohama 226-8501, Japan. E-mail: hmihara@bio.titech.ac.jp; Fax: +81-45-924-5833; Tel: +81-45-924-5756
First published on 24th June 2005
Abstract
Three kinds of biotinylated peptides with different linkers between biotin and β-sheet peptide were designed and synthesized. The transmission electron microscopy revealed that the biotinylated peptides self-assembled to form a tubular structure with external diameter of ca. 60 nm and inner diameter of ca. 30 nm in an aqueous solution. The anti-biotin antibody effectively bound to biotin groups in the peptide nanotubes. The binding of antibody was regulated by not only the concentration of the protein in the solution but also the properties of biotinylated peptides forming the tubes. The antibody preferentially bound to the biotinylated peptide tubes assembled from the peptide with the most hydrophilic linker, suggesting that the surface properties and functions of the tubular structure were modulated and engineered by the design of the peptides.
Biomolecules are excellent in hierarchically organizing a characteristic structure by self-assembly as shown in natural biosystems. The advantage of the spontaneous assembly of biomolecules has advanced a bottom-up approach for fabrication of nanostructures and nanomaterials.1–7 Peptide-based assemblies are notable because of the flexibilities of design and synthesis for modulating the molecular assembly and mounting functions.8–12 We have previously reported the fabrication of peptide fibers with a uniform width of about 100 nm using designed short β-sheet peptides composed of 10 amino acid residues.13 The peptide fibers, especially self-assembled from the peptide FI (the amino acid sequence; PKFKIIEFEP), exist as an individual single fibers and do not form a gel at peptide concentrations of less than several millimolar in aqueous solutions near neutral pH. The single straight fiber with high regularity and uniformity potentially provides an appropriate scaffold for arranging functional molecules in a regulated manner on fabricated fibers, leading ultimately to the development of functional nanomaterials. Here, we have attempted the modification of 100 nm-sized peptide fibers with proteins using biotinylated designed peptides.
We have introduced three kinds of linkers of different hydrophobicity between biotin and the N-terminus of the β-sheet peptide FI, because the linkers are considered to largely affect the peptide assembly. Also, we have supposed that the linkers would generate different surface properties of peptide fibers to control the binding of proteins to biotin groups in the fibers.
The biotinylated peptides consisted of three parts; biotin, a linker, and a β-sheet forming region FI (Fig. 1). The most hydrophobic linker HH was composed of two units of 6-aminohexanoic acid, and the most hydrophilic linker DD was two units of 8-amino-3,6-dioxaoctanoic acid. The middle linker DH was 8-amino-3,6-dioxaoctanoic acid linked with 6-aminohexanoic acid. The peptides were synthesized by the standard 9-fluorenylmethyloxycarbonyl (Fmoc) solid phase synthetic method, and at the N-terminus the linker amino acid and biotin were introduced on the resin. Each of the prepared three kinds of biotinylated peptides, BiHH-FI, BiDD-FI, and BiDH-FI was dissolved at the concentrations of 1–3 mM in water containing ethanol (0–20%), and sonicated at 50 °C. After several hours, the solution was cooled down to room temperature, allowing the assembled peptides to be observed visually in each peptide solution.
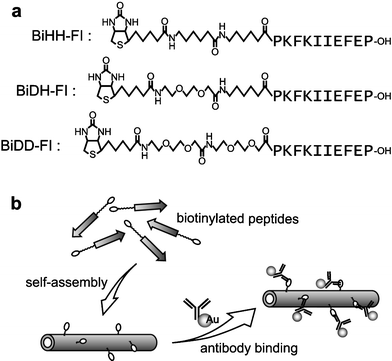 | ||
| Fig. 1 (a) The structures of the designed biotinylated peptides. (b) Schematic illustration of the formation of biotinylated peptide tubes and the modification with anti-biotin antibodies labelled with colloidal gold. | ||
The peptide assemblages were studied by transmission electron microscopy (TEM) after negative staining with 2% (w/v) phosphotungstic acid. It was revealed that each of the biotinylated peptides formed a tubular structure (Fig. 2a and 2b). The TEM images suggested that the peptide nanotubes were highly homogeneous, having uniform external diameter of 50–70 nm and inner diameter of 20–35 nm. The thickness of the wall was around 15 nm. All three kinds of biotinylated peptides formed similar diameter tubular structures, and there were no significant differences in their TEM images. Atomic force microscopy (AFM) showed the peptide assemblages as straight and having a width of around 100 nm (Fig. 2c), closely resembling the images observed by TEM. The cross section of the tube showed that the peptide tubes had a height of about 20 nm, and also that the center of the tubes was slightly depressed (Fig. 2d). The central indent indicated that the tubular structure formed in water was depressed by the adsorption on the surface and a dry condition for the AFM experiment. Considering that the height of the tube corresponded to the doubled layers, the thickness of the single layer was estimated to be about 10 nm. This thickness was approximately consistent with that of the wall of the tubes estimated from the TEM images.
 | ||
| Fig. 2 The images of the tubular structure assembled from biotinylated peptide BiHH-FI. (a and b) The TEM images of the peptide assemblages negatively stained with phosphotungstic acid. Scale bars = 100 nm. (c) The phase images obtained by tapping-mode AFM on HOPG. (d) The cross section of the peptide tube in the image of (c). | ||
The FTIR spectroscopy provided information on the peptide secondary structure in the tubes. FTIR spectra for all peptide tubes assembled in D2O showed an amide I′ band with a maximum at 1620 cm−1, suggesting that the peptide tube was rich in β-sheet structure.14,15 Additionally, an observed weak peak at 1686 cm−1 indicated that the β-sheet had an antiparallel conformation. The characters observed in the amide I′ region for these biotinylated peptides were closely similar to that of the peptide fibers formed from non-biotinylated peptide FI, which was composed of only 10 amino acid residues. Therefore, the peptide fibers of FI and the tubes of the biotinylated peptides with hydrophobic and/or hydrophilic linkers likely had the same secondary structure in the amino acid region. The peptide fibers of FI were previously estimated by TEM and AFM to be in a tightly coiled ribbon, and the width of the fibers was 80–120 nm. When the previously observed FI fibers were greatly deflated ones of the tubular structure and the circumference of the tube was about 200 nm, the diameter of the virtual FI tubes would be about 65 nm.13 This value for the diameter roughly corresponded with the observed external diameters of the biotinylated peptide tubes (50–70 nm estimated by TEM images) in this study. In the biotinylated peptides, we found images indicating coiled ribbons like the FI fibers of the tightly coiled left-handed helical ribbon (data not shown).13 It can be speculated that both the biotinylated and non-biotinylated peptides adopted similar structures from the secondary structure to the macrostructure as a tube, that is, antiparallel β-sheets assembled to make up a ribbon which helically coiled to form a tubular structure, as reported previously.13 In the tubes of biotinylated peptides, the height of a single layer was estimated to be about 10 nm, thicker than that of the FI fibers (2–4 nm).13 Although the added linkers might contribute to the structure formation such as the thicker wall of the tube structure, the self-assembled structures were mainly regulated by the amino acid sequence which prefers antiparallel β-sheets.
Moreover, we have attempted to use the tubes of biotinylated peptides as a scaffold for arraying proteins. Colloidal gold-labelled proteins enabled one to visualize by TEM the proteins bound to the peptide tubes.16† In the previous study, using a differently designed peptide composed of 17 residues, we have demonstrated the binding of streptavidin to the thin peptide fibrils with a width of ca. 10 nm by co-assembly of peptides including biotinylated ones.17 In the present study, the co-assembly of peptide FI and the biotinylated one provided peptide fibers with a 100 nm-width, the same as the peptide FI. The co-fibers were, however, rather inactive for streptavidin binding, probably due to the higher ordered and well-packed structure compared to the fibrils with a width of 10 nm. To functionalize the 100 nm-sized regular fibers, making the homogeneous fibers only from biotinylated peptides was available. Anti-biotin antibody was effectively bound to the tubes of the biotinylated peptides, although streptavidin could not bind to the tubes effectively, probably due to the peptide packing and surface differences of these tubes from those of the previous thin (10 nm) fibers. Thus, anti-biotin antibody was used for the subsequent experiments with the nanotubes. As shown in Fig. 3, the anti-biotin antibody apparently bound to the biotinylated peptide tubes. In contrast, the antibody did not bind to the tubes of non-biotinylated peptide FI. The amount of the bound antibodies to the biotinylated tubes was dependent on the concentrations of the antibody in the incubation buffer (Fig. 3e), indicating the specific binding of the antibody to the biotinylated tubes. When the higher concentration of antibody (3.1 µg mL−1) was used, no significant difference in the antibody binding by a variety of the linkers was observed (Fig. 3e). In the trial using the antibody at the concentration of 10 µg mL−1, the frequency of gold particles bound to the peptide tubes was of a similar degree to that using the antibody at 3.1 µg mL−1 (data not shown), indicating that the antibody binding was almost saturated at such concentrations. However, the antibody binding was regulated by the different linkers in the peptides using the lower concentration of antibody (0.31 µg mL−1). Of the three kinds of biotinylated peptides, BiDD-FI with the most hydrophilic linker was most preferred for binding by antibody, whereas BiHH-FI with the most hydrophobic linker was the least preferred. If a non-specific hydrophobic interaction mainly contributed to the antibody binding to the peptide tubes, BiHH-FI with two pentyl units would be superior to the peptides with glycol ether unit(s). We confirmed that, similar to the FI tubes, the antibody did not bind to the tubes made from the peptide with the hydrophobic HH linker, which was acetylated at the N-terminus instead of biotin (AcHH-FI).16† These results indicated that non-specific hydrophobic binding of the antibodies was effectively suppressed under the conditions, and that the antibody specifically bound to biotin in the peptide tubes. Because the hydrophilic linker amino acid, 8-amino-3,6-dioxaoctanoic acid, is longer than the hydrophobic amino acid, 6-aminohexanoic acid, the distanced biotin groups from β-sheets that is the core of fiber structure would be of advantage to supplying the biotin on the tube surface in the case of BiDD-FI. In addition, BiHH-FI might be of disadvantage because hydrophobic alkyl linkers generally tend to be buried inside and to be densely aligned. Although the apparent difference in the tubular structures made from each of biotinylated peptides could not be observed in our experiments demonstrated here, there might be some variation in the surface structure, especially around the biotin groups. Some flexibility around biotin on the tube surface conceivably improved the binding of antibody to the peptide tubes. The peptide packing and surface modification of β-sheet fibers and tubes may be critical factors for their manipulation.
 | ||
| Fig. 3 Modification of the peptide tubes with colloidal gold-labelled anti-biotin antibody. (a) The TEM image of the peptide tubes of FI with the higher concentration of antibody (3.1 µg mL−1). The TEM images of the peptide tubes of BiHH-FI (b) and BiDD-FI (c) with the lower concentration of antibody (0.31 µg mL−1). (d) The TEM image of the peptide tubes of BiHH-FI with the higher concentration of antibody (3.1 µg mL−1). (e) Average number of bound antibody per 1 µm of peptide tubes of BiDD-FI (black bar), BiDH-FI (gray bar), and BiHH-FI (white bar). Goat anti-biotin IgG antibody was labelled with colloidal gold (the diameter; 10 nm). Scale bars = 100 nm. | ||
In conclusion, we have demonstrated the construction of highly homogeneous peptide nanotubes using designed β-sheet peptides with a biotin group. The decoration of the peptide tubes with proteins was effectively achieved using the binding of anti-biotin antibody to biotin groups displayed on the peptide tubes. Accumulation of the antibody on the peptide tubes was regulated by not only the concentration of antibody but also the structural difference of the peptide tubes induced by the characters of the linkers between biotin and β-sheet motif. These studies imply that the design of peptide monomers can modulate the surface or structure of the peptide tubes and regulate the modification on the tubes with proteins. The assembled nanostructure of designed peptides can be engineered as a desirable base for functional nanomaterials.
References
- X. Zhao and S. Zhang, Trends Biotechnol., 2004, 22, 470–476 CrossRef CAS.
- C. Viney, Curr. Opin. Solid State Mater. Sci., 2004, 8, 95–101 CrossRef CAS.
- N. C. Seeman, Biochemistry, 2003, 42, 7259–7269 CrossRef CAS.
- M. Sarikaya, C. Tamerler, A. K.-Y. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577–585 CrossRef CAS.
- C. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, Science, 2004, 303, 213–217 CrossRef CAS.
- C. M. Niemeyer, Angew. Chem. Int. Ed., 2001, 40, 4128–4158 CrossRef CAS.
- H. Hess, G. D. Bachand and V. Vogel, Chem. Eur. J., 2004, 10, 2110–2116 CrossRef CAS.
- M. G. Ryadnov and D. N. Woolfson, J. Am. Chem. Soc., 2004, 126, 7454–7455 CrossRef CAS.
- E. Genové, C. Shen, S. Zhang and C. E. Semino, Biomaterials, 2005, 26, 3341–3351 CrossRef CAS.
- V. Kayser, D. A. Turton, A. Aggeli, A. Beevers, G. D. Reid and G. S. Beddard, J. Am. Chem. Soc., 2004, 126, 336–343 CrossRef CAS.
- M. O. Guler, S. Soukasene, J. F. Hulvat and S. I. Stupp, Nano Lett., 2005, 5, 249–252 CrossRef CAS.
- H. Matsui, P. Porrata and G. E. Douberly, Jr., Nano Lett., 2001, 1, 461–464 CrossRef CAS.
- S. Matsumura, S. Uemura and H. Mihara, Chem. Eur. J., 2004, 10, 2789–2794 CrossRef CAS.
- G. Zandomeneghi, M. R. H. Krebs, M. G. Mccammon and M. Fändrich, Protein Sci., 2004, 13, 3314–3321 CrossRef CAS.
- J. P. Schneider, D. J. Pochan, B. Ozbas, K. Rajagopal, L. Pakstis and J. Kretsinger, J. Am. Chem. Soc., 2002, 124, 15030–15037 CrossRef CAS.
- Experimental details and TEM images are included in the Electronic Supplementary Information.
- H. Kodama, S. Matsumura, T. Yamashita and H. Mihara, Chem. Commun., 2004, 2876–2877 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: modification of peptide tubes with antibody. See http://dx.doi.org/10.1039/b504516a |
| ‡ Present address: Department of Protein Engineering, Cancer Institute, Japanese Foundation for Cancer Research, 3-10-6 Ariake, Koto-ku, Tokyo 135-8550, Japan. |
| § Present address: Department of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan. |
| This journal is © The Royal Society of Chemistry 2005 |
