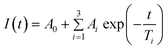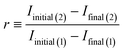The autofluorescence of plastic materials and chips measured under laser irradiation
Aigars
Piruska
a,
Irena
Nikcevic
a,
Se Hwan
Lee
b,
Chong
Ahn
b,
William R.
Heineman
a,
Patrick A.
Limbach
a and
Carl J.
Seliskar
*a
aDepartment of Chemistry, University of Cincinnati, PO Box 210172, Cincinnati, OH 45221, USA. E-mail: carl.j.seliskar@uc.edu; Fax: +1 513 556 9239; Tel: +1 513 556 9213
bDepartment of Electrical and Computer Engineering and Computer Science, University of Cincinnati, PO Box 210030, Cincinnati, OH 45221, USA
First published on 1st November 2005
Abstract
Plastic materials have the potential to substitute for glass substrates used in microfluidic and μTAS systems adding flexibility in materials' choices. Optical quality plastic materials with a low autofluorescence are crucial for optimal detection by fluorescence and laser induced fluorescence techniques. This paper summarizes a series of optical investigations on commercially available plastic chip materials (PMMA, COC, PC, PDMS) and chips made from those materials. Intrinsic optical constants of plastic materials—refractive index for bulk materials—determined by spectroscopic ellipsometry and transmission spectroscopy in the visible range are presented. The laser-induced autofluorescence of materials and chips was assessed at four laser wavelengths, namely, 403, 488, 532 and 633 nm. Considerable bleaching of the autofluorescence was observed under continuous laser illumination. Overall, the longer wavelength laser excitation sources yielded less autofluorescence. PDMS exhibited the least autofluorescence and was comparable to BoroFloat glass. In all cases, chips exhibited slightly higher autofluorescence than the raw plastic materials from which they had been made.
Introduction
There can be little doubt that plastic materials are of growing importance in the fabrication of microfluidic chips and several reviews have been devoted to their properties and fabrication processes.1–3 Plastic materials offer cheap, commercially available alternatives to glass for chip fabrication and are amenable to a wide variety of chip production techniques. In applications where on-board optical absorption or fluorescence is chosen as a means of detection, the optical properties of the plastic materials themselves become important. It is well known that plastic materials show significant fluorescence background or autofluorescence when excited by near-UV or even visible radiation.4–6 Additives in plastic formulations can introduce both strongly absorbing near-UV and new fluorescent species into them. Autofluorescence interferes with on-chip optical measurements and often leads to suboptimal limits of detection.Only a few reports have been devoted to autofluorescence in plastic materials. Hawkins and Yager4 studied the autofluorescence of various plastic materials with broadband light source excitation. Continuous illumination led to a reduction of autofluorescence with complex kinetics over the period of hundreds of minutes. Even though autofluorescence can be reduced under these conditions, the slow autofluorescence reduction (bleaching) made it impractical to use as a chip pretreatment. These authors concluded that polycarbonate, poly(methylmethacrylate) (PMMA, Rohaglas) and polyolefine (Topas) were the materials with the lowest levels of autofluorescence. Soper and co-workers5,6 have shown that using dye-labeled analytes with laser excitation and time-resolved fluorescence in the near-infrared reduced the influence of autofluorescence on analytical measurements done on-chip. Laser excitations at 488, 680 and 780 nm were used and plastic materials exhibited a lower autofluorescence when excited at the two longer wavelengths. In this case, PMMA and polycarbonate samples exhibited autofluorescence levels comparable to glass.5
Our group is developing high throughput chip-based assays for trace determination and measurements of the physiochemical properties of drug candidates. Assays transferred from reusable glass chips to cheaper disposable plastic chips would eliminate lengthy cleaning steps between analyses and avoid potential cross contamination, thus increasing overall throughput of the process. Due to its excellent sensitivity, laser-induced fluorescence (LIF) is our main detection method for analyses. Even though LIF requires a fluorescent analyte and cannot directly be used as a universal detection method, there are well known dependable techniques for labeling biomolecules with high quantum yield fluorophores.
The purpose of this paper is to report an evaluation of the optical constants and the autofluorescence of several candidate plastic materials and the associated chips that we have made from them. The material autofluorescence under laser excitation at several wavelengths and powers was studied and the kinetics of the bleaching of this intensity have been quantitatively described. Plastic materials are compared to BoroFloat glass, a common substrate for chip fabrication with a low autofluorescence under LIF.
Experimental
Materials
The following commercially available materials were used: 1 mm BoroFloat (Schott), 5.0, 3.0 and 0.25 mm poly(methylmethacrylate) (PMMA, GoodFellow), 0.25 and 2.0 mm polycarbonate (PC, GoodFellow), poly(dimethylsiloxane) (PDMS, Dow Corning Sylgard 184 kit). PDMS sheets were made by mixing base to curing agent at a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (w/w), degassing, pouring into a Petri dish, and degassing again. PDMS sheets obtained were cured on a hotplate for 2 h at 80 °C. 1 mm thick sheets from cyclic olefin copolymer (COC, Topas) were made by injection molding (IM). COC, PMMA (GE Polymerland) and PC (GE Plastic) chips were fabricated using injection molding.7 Additionally, PMMA (GoodFellow) chips were made by hot embossing (HE).
1 ratio (w/w), degassing, pouring into a Petri dish, and degassing again. PDMS sheets obtained were cured on a hotplate for 2 h at 80 °C. 1 mm thick sheets from cyclic olefin copolymer (COC, Topas) were made by injection molding (IM). COC, PMMA (GE Polymerland) and PC (GE Plastic) chips were fabricated using injection molding.7 Additionally, PMMA (GoodFellow) chips were made by hot embossing (HE).
Transmission spectra of materials were recorded on a HP 8453 diode array spectrophotometer and refractive indices determined on a variable angle spectroscopic ellipsometer (J.A.Woollam, Inc.).
Laser-induced fluorescence instrumentation
Material autofluorescence was measured using a Nikon TE 2000 epifluorescence microscope equipped with a H6780-20 PMT module (Hamamatsu) and a CoolSnap HQ CCD camera (Roper Scientific). Current from the PMT was amplified with a SR570 (Stanford Research) low current preamplifier and digitized using a PCI P6036 DAQ card (National Instruments) with software written in LabView (National Instruments). The CCD camera was interfaced with a personal computer through a PCI card and data were acquired using MetaMorph (Universal Imaging Corporation) software. Four lasers (20 mW, 403 nm, IQ2C20 (PTI Inc.); 50 mW, 488 nm, (Melles Griot); 20 mW, 532 nm, 85-GCB-020 (Melles Griot); 35 mW, 633 nm, 25LHP928-249 (Melles Griot)), and a Lambda LS xenon arc lamp (Sutter Instrument Company) were used as light sources. The fluorescence and excitation of the fluorescence were filtered by appropriate laser filter sets (Chroma Inc.) for 405 nm (excitation filter z405/20×; dichroic mirror z405rdc; emission filter hq460/50m), 488 nm (z488/10×; z488rdc; hq525/50m), 532 nm (z532/10×; z532rdc; hq580/60m) and 633 nm (z633/10×; z633rdc; hq685/70m). Laser power was measured at the sample with a PM-300 (Kimmon Electric Co.) power meter.Measurements of material autofluorescence
Prior to analysis, candidate materials were sonicated in ethanol for 10 min. A piece of plastic material or chip sample was placed on the microscope stage and the microscope objective was aligned to give a sharp image of the sample's bottom (closer to objective) surface on the CCD camera. Then, the objective was moved ∼250 µm closer to the sample resulting in a focal point about this distance into the material. This last step ensured that the laser excitation was focused into the bulk of the material. The microscope was then switched to use a Hamamatsu phototube as the detector. Then data acquisition and illumination were started while recording the autofluorescence. Before each new measurement the sample was translated on the sample stage to ensure a fresh unbleached sample spot for illumination. All measurements were done with a 10× microscope objective and at 1, 3 and 5 mW laser power at the sample. For thin samples (0.25 mm sheets) locations of the sample bottom and top surfaces were determined and the objective adjusted to focus light midway between these surfaces.To examine how the autofluorescence changed with microscope objective-to-sample distance the following procedure was used. The sample was placed on the calibrated microscope stage and the positions of the bottom and top sample surfaces located. Then the objective was backed off from the sample (image plane closer to the objective than the sample bottom surface). The objective was then moved toward the sample in 250 µm steps and the autofluorescence signal recorded continuously.
The long term recovery of material autofluorescence was examined in the following way. A sample was aligned and illuminated for 10 min as described above. The same spot was then illuminated for an additional 10 min approximately 12 h later while recording the autofluorescence.
Results and discussion
Optical properties of materials
A fundamental optical property of a material is the complex refractive index, ñ = n + ik, where ñ, n, k are the complex refractive index, the real part, and the imaginary part of the index, respectively. The constant k is proportional to the extinction coefficient of the material and thus gives information on the attenuation of light by the material itself. We have evaluated the refractive index of materials by spectroscopic ellipsometry and values at the laser wavelengths used are summarized in Table 1. The real parts of the refractive index values obtained agree with transmission data presented in Fig. 1. The samples with absorbance bands closer to the visible range (PC and thin PMMA materials) have significantly higher refractive indices. On the other hand, PDMS with high absorbance located furthest in the UV has the lowest refractive index. Additionally we attempted quantitative evaluation of the extinction coefficients at these same wavelengths by globally fitting ellipsometry and transmission data. Several factors, namely, the low optical losses of materials, surface and bulk imperfections, and light source fluctuations prevented us from obtaining reliable values of extinction coefficients in the visible range. Instead, normal incidence transmission spectra of plastic materials are presented in Fig. 1. Typically, 10 to 15% of the losses over the visible range are due to reflections at sample interfaces and these losses increase for higher refractive index value materials. The only exception to this was COC that had the lowest transmission over the visible range. This could be attributed to scattering by surface and bulk imperfections which are considerably more abundant in COC than in any other material studied.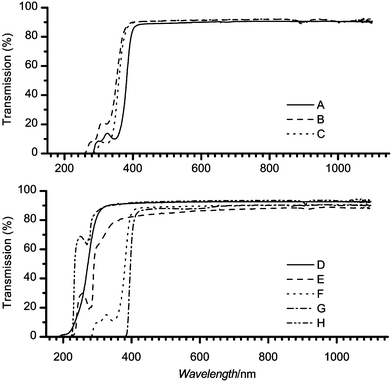 | ||
| Fig. 1 The transmission spectra taken at normal incidence of the plastic materials are shown. Top panel: (A) – 0.25 mm, (B) – 3.0 mm and (C) – 5.0 mm PMMA. Bottom panel: (D) – BoroFloat, (E) – COC, (F) – 0.25 mm and (G) – 2.0 mm PC, (H) – PDMS. | ||
| Sample | λ/nm | ||||
|---|---|---|---|---|---|
| 403 | 488 | 532 | 633 | ||
| Materials | BoroFloat | 1.494 | 1.483 | 1.480 | 1.475 |
| PMMA 0.25 | 1.621 | 1.599 | 1.592 | 1.581 | |
| PMMA 3.0 | 1.495 | 1.490 | 1.488 | 1.486 | |
| PMMA 5.0 | 1.506 | 1.496 | 1.493 | 1.488 | |
| PC 0.25 | 1.620 | 1.598 | 1.591 | 1.580 | |
| PC 2.0 | 1.627 | 1.602 | 1.594 | 1.582 | |
| COC | 1.544 | 1.534 | 1.530 | 1.525 | |
| PDMS | 1.428 | 1.418 | 1.415 | 1.410 | |
| Chips | PMMA IM | 1.510 | 1.498 | 1.495 | 1.489 |
| PC IM | 1.636 | 1.612 | 1.604 | 1.592 | |
Autofluorescence of materials
A typical plastic material autofluorescence time profile under continuous laser illumination is shown in Fig. 2. The points on the graph represent the autofluorescence starting at very early times where one can see a sharp decrease followed by a more gradual decay of the intensity. After a period of a few hundreds of seconds the autofluorescence intensity approaches a limiting value. The autofluorescence intensity generally scales linearly with applied laser power. In contrast the autofluorescence of BoroFloat, used as a reference material, remained essentially unaffected by illumination time (see bottom curve in Fig. 2).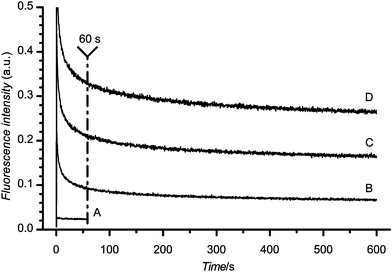 | ||
| Fig. 2 Autofluorescence of COC with 488 nm excitation at 1 (B), 3 (C) and 5 (D) mW laser power. BoroFloat autofluorescence at 1 mW (A) is shown for comparison. | ||
The comparison of the autofluorescence of several materials is shown in Fig. 3. The absolute response of the PMT for BoroFloat at each excitation wavelength is shown in the inset in Fig. 3. The autofluorescence intensity of all materials is indicated relative to the autofluorescence of BoroFloat at a particular excitation wavelength and represents the autofluorescence of the material after 60 s of continuous laser illumination (indicated by vertical line in Fig. 2). The presentation of autofluorescence intensity after 60 s of illumination was chosen for two reasons. First, this time period allowed the autofluorescence to approach steady state. Second, such an illumination period could be used to optically pre-treat the detection spot prior to an analysis.
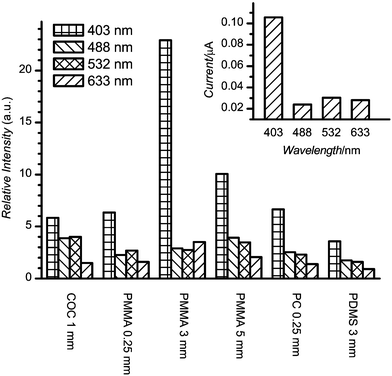 | ||
| Fig. 3 Autofluorescence of plastic materials under 1 mW laser power after 60 s of illumination. Intensity is relative to BoroFloat under the same conditions. The absolute intensity of BoroFloat autofluorescence is shown in the inset. | ||
A few general conclusions follow from these measurements. First, the BoroFloat autofluorescence was slightly higher (∼3 times) at 403 nm than the essentially constant level at other laser excitation wavelengths. Second, for most plastic materials the autofluorescence tended to decrease as laser excitation wavelength increased. The most dramatic decrease was observed in going from 403 to 488 nm laser irradiation. Third, the lowest autofluorescence was exhibited by PDMS. At 633 nm excitation, it was comparable with BoroFloat; at other wavelengths 2 to 3 times higher than BoroFloat. Additionally, PDMS exhibited an essentially constant, invariant to illumination, glass-like autofluorescence. The rest of the materials had autofluorescence levels ∼3 to 5 times higher than BoroFloat, except for 403 nm excitation where the autofluorescence for any plastic material was significantly higher than BoroFloat. Even though the thin 0.25 mm PC was comparable to PMMA and COC materials, the thicker 2.0 mm PC material exhibited significantly higher autofluorescence (not shown in figure). The least difference was observed at 633 nm excitation where 2.0 mm PC autofluorescence exceeded BoroFloat by ∼40 times. The difference was even larger at shorter excitation wavelengths.
The results presented are typical of these plastic materials. In certain cases, considerable spot-to-spot variation of the autofluorescence was observed. To examine the nature of these variations we evaluated how optical alignment with the material influenced the measured autofluorescence. Autofluorescence was recorded varying the vertical positions of the microscope objective relative to the sample. Typical results are presented in Fig. 4. The two vertical lines in the figure represent the top (on the right) and bottom (left) surfaces of sample. The lowest autofluorescence was observed when excitation was focused into the bulk of the material. In this case the autofluorescence intensity was significantly diminished by material bleaching due to the high power density inside the material. The magnitudes of the intensity variations agreed well with the analyses of dynamic data. Thus, the vertical alignment was one of the important factors that affected the autofluorescence signal observed. The sample surface and bulk material defects and illumination history were other factors that influenced the measurement reproducibility.
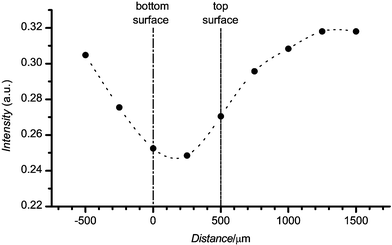 | ||
| Fig. 4 The autofluorescence of COC as a function of distance normal to the surface of the material. ● represents experimentally determined points, the dashed line is provided to guide the eye. The positions of the back and front surfaces of the material are indicated. The horizontal axis indicates the image plane relative to the bottom surface (sample surface closest to objective). The COC physical thickness (nominally 1 mm) is larger than the apparent thickness (∼0.5 mm) because of the difference in the refractive index between air and the plastic material. The apparent thickness is 1/n times the physical thickness, where n is the refractive index of the material. | ||
Autofluorescence of chips
Autofluorescence of chips was evaluated for 4 different chip types: PMMA hot embossed and injection molded, COC injection molded and PC injection molded chips. Typical autofluorescence data for chips under continuous laser illumination were very similar to the materials presented in Fig. 2. Comparison of various chip samples after 60 s of illumination is depicted in Fig. 5. The autofluorescence intensity is given relative to BoroFloat autofluorescence at the same excitation wavelength.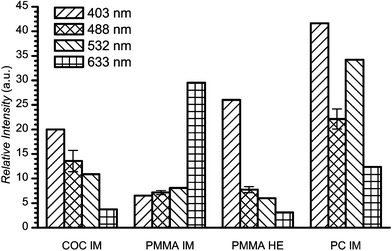 | ||
| Fig. 5 The relative autofluorescence of plastic chips under 1 mW of laser power after 60 s of illumination (intensity relative to BoroFloat). | ||
Overall, the autofluorescence of chips exhibited trends similar to the associated materials. The shorter the laser excitation wavelength, the higher the autofluorescence of chips observed. The only exception was the PMMA injection molded chip that showed a significant increase in autofluorescence when excited at 633 nm. This behavior was unexpected and the cause of this behavior remains unclear to us.
The vertical alignment of the focus point of the laser (discussed in more detail for materials previously) led to significant variation in the autofluorescence measurements. The standard deviation of the autofluorescence signal was determined for all chips at 488 nm excitation and typically was from 10 to 20%. The autofluorescence intensity at 488 nm shown in Fig. 5 is the average of 5 measurements; the associated error bars represent standard deviations for these measurements.
The important thing to note is the difference in autofluorescence intensities between Fig. 3 and 5. In general, all plastic chips studied had higher autofluorescence levels than the associated materials. For most of the materials, autofluorescence was 3 to 5 times higher than BoroFloat. On the other hand chips exhibited approximately 5 to 10 times higher autofluorescence levels than BoroFloat. The PMMA chips showed the lowest autofluorescence levels followed by COC and then PC chips. Due to its physical properties PDMS is not an appropriate material for our applications and no attempt was made to characterize a PDMS chip. Nonetheless, superior chip autofluorescence levels might be anticipated based on our PDMS material studies.
Dynamics of autofluorescence
In general, on laser illumination the autofluorescence of plastic materials and chips decreased until a near steady-state was reached. For practical purposes we chose to characterize this bleaching phenomenon by fitting the experimental decay data to the following empirical expression:where A0 represents the final steady-state intensity of the autofluorescence, Ai, the magnitude of decay components, Ti, the characteristic decay time in seconds, and t the laser illumination time in seconds. This equation is analogous to that used by Hawkins and Yager.4 However, in their work A0 accounted for CCD dark count; in our work the parameter A0 was fitted to indicate the final steady-state autofluorescence level.
For all samples (materials and chips) studied three different decay components were sufficient to characterize the dynamics of the autofluorescence bleaching. The BoroFloat and PDMS materials exhibited the lowest autofluorescence variations and could be fitted with fewer exponential decay terms. Since our main interest was in the properties of fabricated chips, we have chosen to present the data for chips, PDMS, and the reference glass BoroFloat. Even though the mechanical properties of PDMS are not appropriate for our needs, its excellent autofluorescence properties are worth noting. Additionally, the chip dynamic data are a good representation of the autofluorescence dynamics of the materials, the main difference being that materials have relatively lower magnitudes of the fitting terms. Table 2 summarizes the fit parameters for all chips and selected materials along with the associated correlation coefficients and χ2 values to indicate the goodness of the fits.
| Sample | 403 nm | 488 nm | 532 nm | 633 nm | ||||
|---|---|---|---|---|---|---|---|---|
| a χ 2; b R 2; c A 0; d A 1; e A 2; f A 3; g T 1; h T 2; i T 3, this pattern is used throughout the table. | ||||||||
| BoroFloat | 3.1 × 10−5a | 0.635b | 4.9 × 10−7 | 0.441 | 7.1 × 10−7 | 0.759 | 6.0 × 10−7 | 0.395 |
| 0.124c | 0.024 | 0.032 | 0.028 | |||||
| 0.038d | 5.6g | 0.003 | 9.2 | 0.006 | 11.6 | 0.003 | 15.1 | |
| —e | —h | — | — | — | — | — | — | |
| —f | —i | — | — | — | — | — | — | |
| PDMS | 6.9 × 10−5 | 0.941 | 7.7 × 10−7 | 0.896 | 1.1 × 10−6 | 0.865 | 5.5 × 10−7 | 0.334 |
| 0.308 | 0.036 | 0.043 | 0.025 | |||||
| 0.182 | 2.0 | — | — | — | — | — | — | |
| 0.122 | 22 | 0.011 | 11 | — | — | — | — | |
| 0.073 | 319 | 0.008 | 170 | 0.011 | 129 | 0.002 | 127 | |
| COC IM chip | 9.3 × 10−4 | 0.996 | 7.2 × 10−6 | 0.997 | 6.9 × 10−6 | 0.998 | 2.1 × 10−6 | 0.989 |
| 1.176 | 0.164 | 0.199 | 0.071 | |||||
| 3.397 | 1.1 | 0.211 | 2.1 | 0.172 | 2.4 | 0.025 | 2.6 | |
| 1.850 | 15 | 0.146 | 24 | 0.148 | 27 | 0.035 | 23 | |
| 1.209 | 178 | 0.106 | 233 | 0.146 | 242 | 0.039 | 222 | |
| PMMA IM chip | 1.8 × 10−4 | 0.990 | 3.8 × 10−6 | 0.995 | 5.2 × 10−6 | 0.997 | 2.6 × 10−5 | 0.998 |
| 0.481 | 0.088 | 0.156 | 0.510 | |||||
| 1.336 | 0.7 | 0.168 | 1.5 | 0.166 | 2.2 | 0.276 | 0.9 | |
| 0.593 | 11 | 0.106 | 17 | 0.127 | 23 | 0.206 | 24 | |
| 0.318 | 153 | 0.061 | 172 | 0.101 | 229 | 0.361 | 307 | |
| PMMA HE chip | 6.7 × 10−4 | 0.998 | 3.4 × 10−6 | 0.991 | 3.2 × 10−6 | 0.995 | 1.8 × 10−6 | 0.971 |
| 1.439 | 0.119 | 0.125 | 0.063 | |||||
| 1.706 | 1.7 | 0.084 | 2.0 | 0.073 | 2.9 | 0.019 | 7.3 | |
| 1.281 | 25 | 0.061 | 21 | 0.066 | 28 | 0.016 | 69 | |
| 1.546 | 214 | 0.048 | 206 | 0.062 | 236 | 0.020 | 515 | |
| PC IM chip | 2.0 × 10−3 | 0.998 | 1.5 × 10−5 | 0.998 | 1.9 × 10−5 | 0.999 | 1.1 × 10−5 | 0.993 |
| 2.511 | 0.277 | 0.698 | 0.286 | |||||
| 5.185 | 1.3 | 0.321 | 2.2 | 0.191 | 4.3 | 0.035 | 3.2 | |
| 3.083 | 17 | 0.234 | 22 | 0.222 | 39 | 0.067 | 34 | |
| 2.234 | 205 | 0.179 | 212 | 0.345 | 333 | 0.125 | 279 | |
It was pointed out earlier that BoroFloat exhibited essentially constant autofluorescence; this is reflected in the fitting parameters. The autofluorescence of BoroFloat could be described by just a single exponential decay term with short lifetime. More importantly, the magnitude is quite small in comparison with other values of the constant term A0. PDMS was the best of the studied plastic materials showing a somewhat similar behavior to BoroFloat. At the two longest wavelengths PDMS has only a single decay term with a low magnitude, but somewhat longer lifetime. For the two shortest wavelengths the results are more typical of the rest of the materials. More exponential decay terms were required and their magnitudes are comparable with the constant term A0.
The correlation coefficients for the fits deviated considerably from 1 for BoroFloat, PDMS and several other materials at longer wavelengths. In all these cases, changes of the autofluorescence were relatively small and, as a result, the magnitude of the constant term A0 dominated the other Ai terms. The changes in the recorded signal were comparable to the noise in the autofluorescence signal, thus yielding poor correlation coefficients despite reasonable fits to the data.
The autofluorescence dynamics of all chips are similar. The fitted decay time constants fall into three broad categories: short (0 to 6 s), medium (10 to 40 s), and long (120 to 500 s) times. A pattern was observed for autofluorescence at different excitation wavelengths. The shorter the excitation wavelength the larger in magnitude were all three decay terms. At 403 nm the total magnitude of the decay terms for any of the chips exceeded by several times the constant term A0. On the other hand, at longer wavelengths this ratio was reduced. Finally at 633 nm the sum of the decay terms was almost equal to the constant term.
Autofluorescence recovery
The permanency of the bleaching of the autofluorescence was studied in the following way. At first, the selected material was illuminated at 488 nm at 1 mW laser power for 10 min. After 12 h in the dark the laser illumination was repeated on exactly the same spot (sample was unmoved between illuminations). Typical results are depicted in Fig. 6. The decay curve on the left represents the autofluorescence during the first laser illumination; the curve on the right shows the autofluorescence during the second illumination. Clearly, the most dramatic changes observed were during the initial illumination. Autofluorescence intensity rapidly decayed and at the end of the first illumination reached essentially a steady-state value. The second exposure to laser light led to similar kinetics except for a marked difference in the magnitude of the autofluorescence and the associated changes. Although the initial autofluorescence under the repeated illumination was considerably less, the steady-state autofluorescence at the end of both illuminations coincided to within experimental error. To quantify this phenomenon we defined an autofluorescence recovery, r, as:where Iinitial and Ifinal represent initial and final autofluorescence intensities, the number in parentheses indicates first or second laser illumination. Using this relationship the autofluorescence recovery for various plastic chips is presented in Fig. 7. Overall, given the uncertainty in the experimental data, the recovery of each material was essentially constant falling into the range 15 to 20% over the 12 h period. It is important to note that despite a modest recovery of the autofluorescence the same low level of autofluorescence could be achieved with a short additional laser irradiation. In turn, this suggests that once plastic chips have their autofluorescence laser bleached, one can use these chips over an extended period of time without significant return of the autofluorescence.
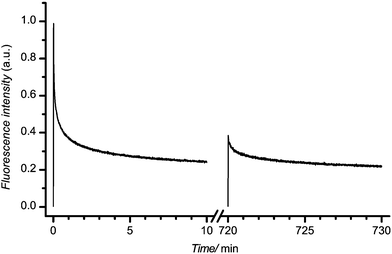 | ||
| Fig. 6 Typical data for autofluorescence recovery over time; PC IM chip illuminated at 488 nm, 1 mW. The curve on the left represents the autofluorescence on initial illumination; the curve on the right, the one recorded after 12 hours of no laser illumination. | ||
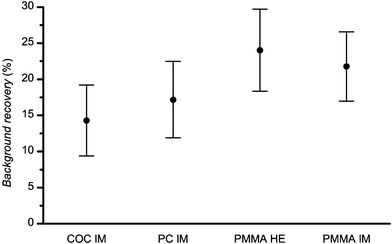 | ||
| Fig. 7 Autofluorescence recovery for plastic microchips. All measurements were performed with 488 nm laser at 1 mW power. For definition of recovery, see text. | ||
Although our immediate interest has been to examine the possibility of quickly laser bleaching the autofluorescence of plastic chips before making analytical measurements, one might speculate as to the origin of the autofluorescence. It seems plausible that the autofluorescence arises from additives and impurities within the plastic materials themselves and, on fabrication, chip processing could introduce more of these. The finding that the autofluorescence bleaches to a steady-state value and recovers somewhat in time suggests that photolytic products of the bleaching might partially recombine restoring some of the initial autofluorescence. However, we hasten to add that one might offer other equally plausible explanations for this interesting and practically important behavior. Given the future of plastic materials in chip fabrication, it would be worthwhile to explore the origin of this behavior with the goal of further reducing the autofluorescence of freshly made chips.
Conclusions
The autofluorescence of BoroFloat glass, several plastic materials and the associated plastic chips were studied at four different laser excitation wavelengths. BoroFloat glass exhibited the lowest, essentially constant autofluorescence at all wavelengths studied. The autofluorescence of plastics, though generally higher than BoroFloat, showed significant changes in magnitude and dynamics. The highest autofluorescence and most severe changes were observed at 403 nm, the shortest wavelength studied. For longer laser wavelengths both the autofluorescence and its changes were reduced. PDMS and PMMA were materials with the lowest autofluorescence. In all cases chips yielded significantly higher autofluorescence levels than the associated materials. Autofluorescence decay under laser irradiation could be quantitatively described using a three component exponential decay function. In turn, these components account for background decays on the order of a few seconds, a few tens of seconds and a few minutes. The laser induced bleaching of the autofluorescence was shown to remain essentially constant up to 12 h after initial laser illumination.Acknowledgements
The authors thank Erik Peterson for help with PDMS materials preparations, Dr Kenneth R. Wehmeyer, Dr Brian H. Halsall and Justin Mecomber for valuable discussions. This work was supported by a grant from the NIH (1 R01 GM069547-01,02) and by an Ohio Board of Regents Doctoral Investment Award.References
- A. de Mello, Lab Chip, 2002, 2, 31N–36N RSC.
- G. S. Fiorini and D. T. Chiu, Biotechniques, 2005, 38, 429–446 CrossRef CAS.
- H. Becker and L. E. Locascio, Talanta, 2002, 56, 267–287 CrossRef CAS.
- K. R. Hawkins and P. Yager, Lab Chip, 2003, 3, 248–252 RSC .★Significant reference.
- M. B. Wabuyele, S. M. Ford, W. Stryjewski, J. Barrow and S. A. Soper, Electrophoresis, 2001, 22, 3939–3948 CrossRef CAS .★Significant reference.
- S. D. Llopis, W. Stryjewski and S. A. Soper, Electrophoresis, 2004, 25, 3810–3819 CrossRef CAS.
- R. Trichur, S. Kim, S. H. Lee, Y. A. Abdelaziez, D. E. Starkey, H. B. Halsall, W. R. Heineman and C. H. Ahn, Micro Total Analysis Systems 2002, Proceedings of the μTAS, Kluwer Academic Publisher, Nara, Japan, 2002, vol. 1, pp. 560–562 Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |