An integrated microfluidic device for influenza and other genetic analyses
R.
Pal†
a,
M.
Yang†
a,
R.
Lin
a,
B. N.
Johnson
a,
N.
Srivastava
a,
S. Z.
Razzacki
a,
K. J.
Chomistek
a,
D. C.
Heldsinger
a,
R. M.
Haque
b,
V. M.
Ugaz
a,
P. K.
Thwar
a,
Z.
Chen
a,
K.
Alfano
a,
M. B.
Yim
a,
M.
Krishnan
a,
A. O.
Fuller
c,
R. G.
Larson
a,
D. T.
Burke
d and
M. A.
Burns
*ae
aDepartment of Chemical Engineering, University of Michigan, Ann Arbor, MI 48109, USA
bDepartment of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, USA
cDepartment of Microbiology and Immunology, University of Michigan, Ann Arbor, MI 48109, USA
dDepartment of Human Genetics, University of Michigan, Ann Arbor, MI 48109, USA
eDepartment of Biomedical Engineering, University of Michigan, Ann Arbor, MI 48109, USA
First published on 18th August 2005
Abstract
An integrated microfluidic device capable of performing a variety of genetic assays has been developed as a step towards building systems for widespread dissemination. The device integrates fluidic and thermal components such as heaters, temperature sensors, and addressable valves to control two nanoliter reactors in series followed by an electrophoretic separation. This combination of components is suitable for a variety of genetic analyses. As an example, we have successfully identified sequence-specific hemagglutinin A subtype for the A/LA/1/87 strain of influenza virus. The device uses a compact design and mass production technologies, making it an attractive platform for a variety of widely disseminated applications.
Introduction
The Human Genome Project has catalyzed research in the area of miniaturized analytical systems, or lab-on-a-chip technologies, for genetic analyses. The development of these systems involves the integration of microfabricated components,1–5 such as microscale separations,6–10 reactions,11–15 microvalves and pumps,16–23 and various detection schemes,24–29 into fully functional devices. Several devices that have integrated DNA amplification reactions and electrophoretic separations have been developed.30–34 Devices that employ hybridization fluorescence detection have been fabricated to perform sample preparation, various fluidic manipulations, and polymerase chain reaction (PCR).35 Integration of multiple steps of biological assays on a single device provides significant advantages in terms of sample/reagent consumption, process automation, analysis speed and efficiency, and contamination reduction.Integrated genetic analysis devices can find applications in two broad categories. The first class is centralized laboratory-based high throughput systems for applications such as genomics, proteomics, metabolomics, and drug discovery.36 These devices are traditionally in the microarray format though there are some exceptions. High throughput systems are designed to yield a large amount of information from a single experiment. They normally are customized, involving complex chemistry/biochemistry processes (e.g., DNA probes for microarrays) in manufacturing. High throughput systems need not be portable and typically require auxiliary equipment for large information processing.
The other class of lab-on-a-chip devices is the group designed for widespread distribution for applications such as infectious disease diagnosis, chemical/biological warfare detection, agriculture pest isolation, and forensic identification.37–41 Unlike the high throughput systems, these devices are typically used to provide extremely specific and simple information about a small number of samples. Widespread dissemination of these devices for point-of-care use by untrained personnel can be one goal and requires a high degree of integration and process automation.
A particular example of a widely disseminated application is the identification of the influenza viral strain. Influenza is a significant long-term public health threat because of its genetic mutability, rapid transmission, and ability to move from species to species.42,43 Influenza pandemics occurred several times in the 20th century (1918, 1957 and 1968), and all had significant death tolls. In each case, a newly assorted influenza strain to which humans have little immunity was introduced into the human population, presumably from an animal source. Recent outbreaks with pandemic potential in East Asia also introduced new H5 avian influenza strains. Given the rapidly changing influenza genome, an adaptable, worldwide surveillance of specific types and subtypes is a high priority,44 and genetic analysis based on PCR and sequence-specific detection provides the most accurate method for typing influenza and other viruses.
We have designed and tested an integrated genetic analysis device. The device is designed to perform two independent serial biochemical reactions, followed by an electrophoretic separation (Fig. 1). The key components (phase change valves,45 thermally isolated reaction chambers,46 gel electrophoresis,47 and pulsed drop motion) that were developed for this device are electronically addressable and simple to operate, properties that can lead to eventual autonomous operation. We have successfully performed influenza viral strain (A/LA/1/87 and A/Sydney/5/97) subtyping on this device using a PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) analysis that distinguished the polymorphism in the hemagglutinin coding region. Other PCR-based analyses including amplification from human genomic DNA (D1S80 locus) and mouse plasmid DNA (SNRPN locus) were also performed.
 | ||
| Fig. 1 (a) Schematic representation of an integrated microfluidic device. There are three liquid entry channels (“L”, sample, PCR reagents and RD reagents), several metering channels, drop mixing intersections, a sealed PCR chamber, an open RD chamber, and an electrophoresis channel. Each valve (“V”) is individually and electronically addressable. (b) Photograph of an assembled device (1.5 cm by 1.6 cm). The discrete liquid drops controlled in this device are 100 to 240 nl, with fluidic channel dimensions of 200–600 µm wide and 50 µm deep. The electrodes and diodes shown in the figure were not used in this work. | ||
Materials and methods
Glass microfabrication
The microfabricated devices are assembled by bonding silicon and glass substrate components (Fig. 2). Borofloat glass wafers (500 µm thick, 10 cm diameter) are annealed at 560 °C for 1 h. Chromium and gold (500/3500 Å) are evaporated on the glass wafer. Photoresist (Microposit SC 1827; Shipley Co., Marlborough, MA) is spun on at 3000 rpm and soft baked at 100 °C for 5 min. The fluidic channel pattern is then exposed on the wafer using a mask aligner (Cannon PLA 501FA, 405 nm wavelength, 350 light integral). The lithography is finished by developing the wafer in MF319 (Shipley Co.) for 1.5 min. The photoresist is hard baked at 135 °C for 30 min. The gold is etched for 4 min and the chromium is etched for 2 min, each in its respective etchant (gold etchant TFA; Transene Co and chromium etchant CR-14; Cynatek Corp., Fremont, CA). The glass is etched in hydrofluoric acid (49% HF, CMOS grade; J.T. Baker, Philipsburg, NJ) to obtain the desired channel depth using an estimated etch rate of 7 µm min−1. The access holes in the wafer are laser drilled (Accu-Tech Laser Processing Inc., San Marcos, CA). In some cases the wafer is coated with a 2 µm thick parylene layer by chemical vapor deposition (PDS 2010 LABCOTER® 2; Specialty Coating Systems, Indianapolis, IN). The glass wafer is diced to obtain the individual devices. | ||
| Fig. 2 Process steps for the fabrication of the hybrid glass–silicon device. (a) Silicon side fabrication; (b) glass side fabrication. | ||
Silicon microfabrication
Silicon fabrication begins by growing a 2000 Å thick thermal oxide on a silicon wafer (〈100〉, 500 µm thick). Lithography for the heater elements involves spinning, soft baking, exposing, developing the photoresist, and is similar to the glass fabrication, except that titanium and platinum (Ti/Pt; 300/1000 Å) are evaporated on the wafer after the resist is patterned. The wafer is left in acetone (CMOS grade; J.T. Baker) for 15 min to liftoff unwanted metal. A 5 µm thick parylene layer is deposited on the wafer by CVD. Lithography for the electrical contact openings is performed and the parylene is etched using oxygen plasma reactive ion etching (RIE 2000; South Bay Technologies, 100 W and 100 mT oxygen plasma). The silicon side is coated with a protective layer of photoresist and diced to obtain the individual devices.Glass–silicon device assembly
A custom designed and mass produced printed circuit board (PCB, Advanced Circuits, Aurora, CO) serves as the platform for electrical connections. After receiving a supply of PCBs, each one has an area removed from the center (using a small router bit or scroll saw) to allow access to the back side of device for active cooling. The diced silicon devices are cleaned (with acetone, isopropyl alcohol, and de-ionized water) and fixed onto the PCB using standard quick cure epoxy. The devices are wire bonded (Kulicke & Soffa 4124 Ball Bonder) using 1.0 mil gold wire. The cleaned glass device component is visually aligned to the silicon side and UV curable optical glue (NOA 72; Norland Products, Cranbury, NJ) is wicked between the glass and silicon sides through the edges. The device is cured in UV light (365 nm) for 6 h and then in an oven at 50 °C for 12 h. The wirebonds are encapsulated with a non-fluorescent epoxy (EP939; Thermoset, Indianapolis, IN). O-rings are attached to each end of the separation channel to act as buffer reservoirs.Pulsed drop motion
Lab air supply, controlled by a pressure regulator (Matheson Gas Products Inc, Irving, TX, Model Number 3701), provides the pneumatic pressure used to move drops in the microchannel. The regulated air that outlets from the pressure regulator is connected via a solenoid valve (Numatics Inc., Model Number LS02L6H00B) to a pipette tip. The final connection to the chip is made by either epoxying or manually holding a pipette tip at the appropriate access hole in the glass device. The solenoid valve is in a normally closed position and may be opened by applying a +12 V DC voltage. Opening and closing of the solenoid valve are controlled through a combination of a DC power supply (Electro Industries, Model Digi 35A) and a relay board (National Instruments, TX, Model ER-16). Functioning of the relay board is controlled through a program written in LabVIEW (National Instruments, TX) and a Digital I/O card (PCI-DIO-96). The pulsed pressure system is also used to operate the phase change valves. The phase change material used in the valves is a custom mixture of M1595 microcrystalline wax and Lycojet wax.47Temperature control
The setup for performing PCR and restriction digest thermal reactions consists of a DC power supply (B+K Precision Model 1760, Yorba Linda, CA), two data acquisition (DAQ) boards (National instruments PCI 6031E and PCI-6704, Austin, TX), two connector blocks (National instruments SCB-100 and SCB-68, Austin, TX), a custom signal conditioning circuit, a computer and two LabVIEW programs (National instruments, Austin, TX). To calibrate the temperature sensors the entire device is incubated in a convection oven whose temperature is ramped from room temperature to 100 °C. The resistance is recorded in a LabVIEW program by sending a current of 3 mA through two leads of the four point sensors with the voltage being measured across the other two leads. The slope and intercept from a linear fit of the resistance and temperature data is read into the control algorithms for temperature control. Temperature is controlled by regulating the voltage across the microfabricated heaters using a proportional-integral (PI) algorithm. The heaters are connected to the power supply through the signal conditioning circuit that boosts the supply voltage from the computer with an op-amp gain of 3. Thermal isolation of the PCR chamber from other components on the device is achieved by removing the PCB under the areas requiring low temperatures and refilling the cavity with a high-conductivity silicone-based thermal grease (Product #54013, AOS Thermal Compounds, Eatontown, NJ). During the operation, the entire device is placed on a probe station or a Peltier device with temperature maintained at 10 °C.Influenza DNA amplification
The PCR-RFLP assay used for the identification of influenza viral strain A/LA/1/87 consists of two biochemical reactions: a PCR followed by a restriction digestion. The hemaggultinin gene (HA1) region of influenza viral RNA was reverse transcribed, PCR amplified, ligated into pGEM-T vector, and cloned into E. coli. The cloned plasmid was used to synthesize RNA in vitro with T7 RNA polymerase. The RNA samples are then subject to reverse transcription to produce the DNA samples that were used in the device. For the on-chip influenza reaction, the PCR primers amplify a 690 bp fragment of the coding region from the cloned, transcribed HA1 RNA, following reverse transcription. The reaction mixture (∼240 nl) consists of 2 ng µl−1 DNA template, 0.2 mM each dNTP, 60 mM Tris-HCl, 15 mM NH4SO4, 1.5 mM MgCl2, 0.1% TX-100, 0.3 mM each primer, and Taq DNA polymerase 75 units ml−1 (Invitrogen, Carlsbad, CA). The thermocycling protocol applied to the device consists of 92 °C for 30 s, and then 35 cycles of the following: 92 °C for 5 s, 55 °C for 10 s, and 72 °C for 20 s, and finally 72 °C for 60 s, for a total cycling time of 22 min. A portion of the PCR product (∼60 nl) is subsequently subjected to a restriction endonuclease digestion within the same device. The digestion reaction mixture consists of 500 units ml−1HpaI restriction endonuclease (New England Biolabs, Beverly, MA), 1X NEBuffer 4, 10 mg ml−1 bovine serum albumin (BSA), and 0.01 mM YOYO-1 fluorescent dye (Molecular Probes Inc., Eugene, OR). Successful HpaI endonuclease digest cuts the PCR product into two smaller fragments of 145 bp and 545 bp. The restriction digest reaction is performed at 37 °C for 10 min. A 690 bp fragment of the hemagglutinin coding regions of influenza A/Sydney/5/97 DNA can be amplified by the same primer set as the A/LA/1/87 strain, but is not digested with HpaI endonuclease.Human and mice DNA amplification
Amplification of the human genomic DNA uses a commercial amplification kit (EDVO-Kit# 334, EDVOTEK, West Bethesda, MD). The template for this reaction is an anonymous human genomic DNA sample. The amplicon is approximately 650 bp. The reaction mixture consists of 2.5 ng µl−1 human genomic DNA, 0.2 mM each dNTP, 1X Taq DNA polymerase buffer, 1.5 mM MgCl2, 0.5 mM each primer, and Taq DNA polymerase 100 units ml−1 (Invitrogen, Carlsbad, CA). The reaction mixture is subject to thermocycling at 94 °C for 5 s, 65 °C for 10 s, and 72 °C for 20 s for 35 cycles. The template for the mouse plasmid DNA amplification is mouse Snrpn gene DNA cloned from genomic Mus spretus into a pGEM vector. The amplicon is 996 bp. The same PCR reaction mixture composition and thermocycling parameters have been used for mouse Snrpn PCR as for Influenza PCR except the genomic templates and primers. The PCR primers used to amplify each genetic marker are presented in Table 1, along with the expected product sizes.| Assay | Forward primer | Reverse primer | Product |
|---|---|---|---|
| Influenza | 5′-GTTTGTTTCTCT GGTACATTCCGC-3′ | 5′-CAACTGTTACCC TTATGATGTGCC-3′ | 690 bp |
| Human D1S80 | 5′-GAAACTGGCCTCCA AACACTCCCCGCCG-3′ | 5′-GTCTTGTTGGAGAT GCACGTGCCCCTTGC-3′ | ∼650 bp |
| Mouse SNRPN | 5′-GACACCAAGAG GTGGTTAAAGC-3′ | 5′-AGCTTGCAGGT ACACAATTTCA-3′ | 996 bp |
Microgel electrophoresis
Electrophoretic separations were performed using photo-polymerizable crosslinked polyacrylamide (ReproGel, Amersham Pharmacia Biotech). Two gel formulations were used: ReproGel High Resolution (8%T) and ReproGel Long Read (6%T). The gel solution was prepared by mixing one part of the acrylamide stock solution with two parts of the photoinitator solution, and is loaded into the channel network through the inlet hole at one end of the separation channel. Gel casting was performed by placing the device under a UV lamp for a period of 10 min using a previously described masking procedure which allows the gel interface to be precisely positioned inside the electrophoresis channel.47 An electric field of around 26 V cm−1 was used for the separation. In all separations, no additional surface treatment of the microchip glass channels was applied. A solution of 1.0X Tris-borate-EDTA (Bio-Rad, Hercules, CA, USA) was used as the running buffer. All reagents were used as received from the manufacturers.Image processing
The migrating DNA bands were detected by their fluorescence using an Olympus SZX-12 fluorescence stereoscope with a mercury arc illumination source, and a CCD camera (Hamamatsu C2400-08 SIT, Hamamatsu Corporation, USA, Bridgewater, NJ) for imaging. The camera output was recorded, digitized, and intensity profiles corresponding to the migrating bands were obtained by extracting the variation in fluorescence intensity with time at a fixed location in the gel using Transform 2D image analysis software (Research Systems, Inc., Boulder, CO). Snap shots of the gel were digitized to obtain the electropherogram (intensity profile along the channel). Control DNA fragments added to the reaction mixture serve as internal size standards.Results and discussion
Integrated genetic analysis device
The integrated genetic analysis device (Fig. 1) performs two temperature controlled serial reactions followed by a gel electrophoresis. The overall operation of the device, shown in Fig. 3, begins with loading specified amount of reagents and sample through the inlet ports (L1, L2, and L3). The test sample and PCR reagents are mixed and positioned in the PCR chamber. The reaction chamber is then sealed by flanking phase-change valves (V3 and V5). The solution is thermocycled using integrated thin film heaters and temperature sensors under the PCR chamber. After thermal cycling, the valves are opened and the reaction product is moved forward. A portion of the PCR reaction product is mixed with an equal volume of the restriction digest reaction reagent (loaded through L3) and positioned in the restriction digest chamber where the second reaction occurs. The restriction digest reaction product is then moved to the separation section where it is electrophoretically separated. | ||
| Fig. 3 Fluidic operation of the device. (a) Sample, PCR and restriction endonuclease digestion (RD) reagents are loaded. (b) Sample and PCR reagent pumped and mixed, and then positioned in the PCR chamber. The valves flanking the PCR chamber are closed. (c) After the PCR, the valves are opened and the product is pumped and placed alongside the RD reagent. (d) The PCR products and RD reagent are pumped and mixed, and then positioned in the second reaction chamber. (e) After the RD reaction, the product is moved to the gel interface. (f) The product is separated by gel electrophoresis. | ||
As a demonstration, we have used the device for influenza A/LA/1/87 strain identification. Fig. 4 shows an electropherogram of an A/LA/1/87 DNA sample obtained from a complete on-chip analysis. The band pattern obtained after 135 s (separation distance < 1 mm) shows 145 bp and 545 bp bands that are Hpa1 restriction digestion products, and 343 bp and 996 bp bands that are internal markers. This pattern indicates that the original sample for this run was A/LA/1/87 influenza viral DNA. Runs starting with A/Sydney/5/97 viral DNA sample produce the two marker bands and a single amplicon band at 690 bp (data not shown). It should be noted that we worked with viral DNA samples from reverse transcription instead of the viral RNA due to the difficulty of sample handling. In separate experiments we have demonstrated successful RT-PCR starting with viral RNA.
 | ||
| Fig. 4 Intensity profile along the gel of the DNA migration pattern for a fully integrated run for the identification of influenza viral strain A/LA/1/87. | ||
Besides the influenza A/LA/1/87 strain identification, we have tested three other amplification reactions on the device. These include amplification from human genomic DNA (D1S80 locus), mouse plasmid DNA (SNRPN locus), as well as plasmid DNA of influenza viral strain A/Sydney/5/97. D1S80 is a simple sequence repeat polymorphism locus present on human chromosome 1 that contains a 16-nucleotide sequence variably repeated between 16 and 40 times in the human population.46 Similar repeat-based polymorphic loci are used in the most common DNA diagnostic and forensic tests. The mouse Snrpn gene encodes polypeptide N associated with the small nuclear ribonucleoprotein particle, and is involved in the mRNA splicing process. Influenza A/Sydney/5/97 strain has a polymorphism between the hemagglutinin coding regions compared to A/LA/1/87 strain, as mentioned before. Fig. 5 shows the electropherograms of the three amplification reactions with two internal size standards, 343 bp and 462 bp in length. The robustness of the device with different reactions makes the device an ideal platform for various genetic analyses.
 | ||
| Fig. 5 On-chip amplification reactions with off-chip analysis. (a) Human genomic DNA PCR (∼650 bp). (b) Mouse plasmid DNA PCR (996 bp). (c) Influenza A/Sydney/5/97 viral DNA PCR (690 bp). The two control DNA markers used in all runs are 343 bp and 462 bp. The separation was performed on a different device in a microchannel (50 µm deep by 400 µm wide) using an electric field of ∼26 V cm−1. | ||
Fluidic control
To perform such a highly integrated analysis there must be a reliable control of fluidic components on the device. Drop motion and positioning was achieved by using a pulsed-air propulsion system. Regulated air pressure was delivered to the microfluidic chip through a solenoid valve controlled by a computer. Short (∼10 ms) pulses of air at moderate pressures (∼10 psi) were applied to drops to move them through reproducible, incremental distances (Fig. 6). The final position of the drop was monitored visually. As seen from the figure, the distance moved by a water drop varies linearly with the number of pulses and is inversely proportional to the drop length. The average distance moved per pulse for the three different drop lengths of 5 mm, 7.2 mm, and 14.6 mm are 236 µm, 183 µm, and 112 µm respectively with corresponding velocities of 23.6 mm s−1, 18.3 mm s−1, and 11.2 mm s−1. It should be noted that the effective pressure head is only ∼0.1 psi due to the dead volume associated with the pressure delivery lines. | ||
| Fig. 6 Discrete drops were incrementally pumped in a microchannel (50 µm deep, 500 µm wide) using 10 ms pressure pulses at 10 psi (see inset). Three different drop lengths of 5 mm ◆, 7.2 mm ■, and 14.6 mm ▲ were used. | ||
Another level of fluid control is the active hermetic sealing of the channels required during PCR using electronically addressable phase change microvalves.45 These valves use a meltable piston that is mobile in the molten phase but forms a strong seal in the solid state (leak proof to at least 250 psi).45 The reversible phase change is achieved by underlying resistive heaters and the motion of the molten piston is accomplished by pneumatic pressure or vacuum. Fig. 7 shows the operation of two of these valves to seal the PCR chamber. After loading the wax in the valves the reaction mixture is positioned in the PCR chamber (Fig. 7a). The valves are closed by heating the piston and applying pressure pulses (1–2 psi, 0.3 s) at the inlet hole (Fig. 7b). For opening the piston is heated again and vacuum pulses are applied at the inlet hole (Fig. 7c). Once positioned in either the closed or the open state no energy is required to maintain this state making the valves latched and hence extremely energy efficient. Moreover, within a complex integrated device, a single pressure-vacuum supply line and independently addressable heaters can control an arbitrary number of microvalves.
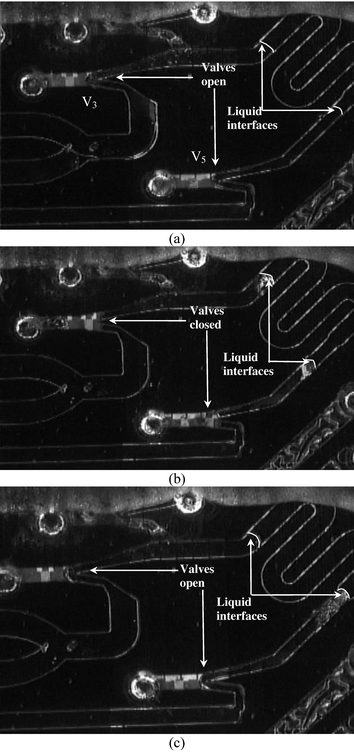 | ||
| Fig. 7 Valve integration with PCR chamber. (a) The reagent is positioned in the PCR chamber. (b) Valves V3 and V5 that flank the PCR chamber are closed (valve V4 is not used and is sealed with UV glue during device assembly). (c) Valves V3 and V5 are opened. Note that there is some evaporation of the reagents during valve operation that can be eliminated by using advanced thermal isolation schemes. | ||
Thermal control
One critical issue associated with operation of the integrated device is temperature control. For a device with a compact design, an accurate as well as localized temperature control is required. Accurate temperature control is achieved by using integrated thin film platinum resistive heaters and temperature sensors because they can be placed spatially close to the reaction chamber, while temperature localization is achieved by using thermal isolation schemes. A typical thermocycling profile is shown in Fig. 8a. To eliminate errors due to lead resistance, a four-wire RTD configuration is used for the temperature sensors. Accurate temperature control for thermocycling (±0.5 °C) is achieved by a LabVIEW PI control module. The reduced thermal mass and the use of silicon substrate allow high heating and cooling rates (both exceed 20 °C s−1). The total reaction time is less than 22 min for 35 cycles (Fig. 8a). Further reduction of the reaction time can be achieved by optimizing the reaction conditions and the temperature control parameters. | ||
| Fig. 8 Thermal control of the integrated device. (a) Temperature profile from the on-chip temperature sensors for 35 cycles controlled by on-chip heaters. The insert shows a blowup of three cycles. (b) Thermal image taken by an infrared camera of a device showing the temperature profile during a PCR thermocycling. The PCR reaction chamber area is heated to 90 °C, while the opposite corner of device remains below 40 °C. The channel layout is overlaid on top of the device to show the position of each component. The dotted line indicates where the underlying PCB has been replaced with a thermally conductive grease. | ||
Isolation of thermally active components (e.g., PCR chamber) from temperature sensitive components (e.g., gel electrophoresis channel) is necessary for the proper functioning of an integrated device, especially for a silicon-based device with a compact footprint.46 For thermal isolation we have used a PCB containing a routed hole filled with a thermally conductive grease, and a 10 °C cold plate or Peltier device below the PCB, as described before. A thermal image taken by an infrared camera of the device during thermocycling shows that the high temperature region is restricted to the PCR chamber (Fig. 8b). Using this technique, the thermal isolation measured by maximum temperature gradient across the device rises to 60 °C cm−1 compared to a device without these isolation strategies (20 °C cm−1). Higher gradients can be achieved using additional thermal isolation strategies such as suspended heaters on a thin diaphragm design.46
It should be noted that silicon has both advantages and disadvantages as a building substrate for integrated devices. The high thermal conductivity of silicon leads to high cooling rates for PCR, with the caveat that thermal crosstalk can be a problem. However, advanced thermal isolation schemes (using deep reactive ion etching (DRIE) etching or chemical wet etching techniques) that combine low power consumption, fast thermocycling and superior thermal isolation can alleviate this problem.46 Silicon can also help to achieve higher level of integration and low cost production. By using a silicon substrate, we have the ability to incorporate active elements such as ion-implanted photodiodes for fluorescence detection on the device,25 or eventually building the microfluidic network on top of CMOS circuits for self-contained operations. Mass production with standard silicon processing technologies, combined with a compact device design, will also significantly reduce the single device fabrication cost, a strategy that has been widely adopted in the microprocessor industry.
Conclusion
The device presented in this work is a step towards building widely disseminated systems for genetic analysis. Analogous to the microprocessor industry, for widely disseminated integrated devices that are also made by mass production, scaling down the device size so that more devices can be accommodated on a single wafer will make these devices cost-effective. The cost per device (currently ∼$7) can be reduced to much less than $1 if we scale down the linear dimensions of the current device by an order of magnitude. This size reduction will allow approximately 100 times more devices to be accommodated on a wafer. Since the phase change valves, microreaction chambers, and separation columns use standard microfabrication technologies, they can be scaled down without significant design modification. We have already tested these components at an order of magnitude reduction in channel dimensions and confirmed that they all function (data not shown). It should also be noted that the separation length used in the present work is an order of magnitude lower than the designed column length (1 mm vs. 1 cm). As the device size becomes smaller, advanced thermal isolation techniques (e.g., silicon DRIE back etching) that can provide excellent thermal isolation are necessary. Thus, a “pennies per chip” cost for this type of design is feasible.Several issues need to be addressed to make the device ready for widely disseminated applications. One of those—sample preparation from either clinical or field isolates—is a significant issue. Different types of sample sources (serum, nasal wash, skin, animal products, etc) will each require specific processing to yield nucleic acid (RNA or DNA) suitable for analysis. However, it is not clear whether this processing will occur before the sample is placed on the chip or in the chip itself. Both procedures are possible – commercial sample preparation systems are currently available for macro scale processing, and microfabricated sample preparation systems have been reported.35,49–55 Our current system assumes that nucleic acids will be purified off-chip allowing for more versatility in the source of the sample but a sample processing unit can be integrated into our design. The choice of which route is optimal will most likely be application specific.
Other issues that need attention are related to portability of the entire unit. Components such as on-chip thermopneumatic pumping can be used to eliminate the need for any pneumatic connections,22 and on-chip photodiodes can be used to replace the external CCD camera.25 Issues such as packaging and micro-macro interfacing also need to be addressed before the device design can be made ready for wide-spread use. While these and other issues must be addressed, the progress reported here provides a substantial step to the end goal.
Acknowledgements
The authors would like to gratefully acknowledge the funding of this work through several grants from NHGRI (most recently P01-HG001984) and a grant from NIAID (R01-AI49541) at the National Institutes of Health. The research team would like to acknowledge Jennifer L. Chisa, Jodi Wilkowski, Scott Ottolini, Vijay Namasivayam, Hao Chen, Sarah Ballough, John Decker Ringo, and Alex Thibonnier for their assistance with reagent preparation, device fabrication, computer programming, lab maintenance, and electronic interface development.References
- K. F. Jensen, AIChE J., 1999, 45, 2051 CrossRef CAS.
- J. P. Landers, Anal. Chem., 2003, 75, 2919 CrossRef CAS.
- D. R. Reyes, D. Iossifidis, P. A. Auroux and A. Manz, Anal. Chem., 2002, 74, 2623 CrossRef CAS.
- P. A. Auroux, D. Iossifidis, D. R. Reyes and A. Manz, Anal. Chem., 2002, 74, 2637 CrossRef CAS.
- E. Verpoorte, Electrophoresis, 2002, 23, 677 CrossRef CAS.
- V. M. Ugaz, R. D. Elms, R. C. Lo, F. A. Shaikh and M. A. Burns, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 1105 CrossRef CAS.
- S. R. Liu and A. Guttman, Trends Anal. Chem., 2004, 23, 422 CrossRef CAS.
- J. Khandurina and A. Guttman, Curr. Opin. Chem. Biol., 2003, 7, 595 CrossRef CAS.
- C. W. Kan, C. P. Fredlake, E. A. S. Doherty and A. E. Barron, Electrophoresis, 2004, 25, 3564 CrossRef CAS.
- C. A. Emrich, H. J. Tian, I. L. Medintz and R. A. Mathies, Anal. Chem., 2002, 74, 5076 CrossRef CAS.
- M. A. Burns, C. H. Mastrangelo, T. S. Sammarco, F. P. Man, J. R. Webster, B. N. Johnson, B. Foerster, D. Jones, Y. Fields, A. R. Kaiser and D. T. Burke, Proc. Natl. Acad. Sci. USA, 1996, 93, 5556 CrossRef CAS.
- M. U. Kopp, A. J. De Mello and A. Manz, Science, 1998, 280, 1046 CrossRef CAS.
- M. A. Northrup, B. Benett, D. Hadley, P. Landre, S. Lehew, J. Richards and P. Stratton, Anal. Chem., 1998, 70, 918 CrossRef CAS.
- M. A. Shoffner, J. Cheng, G. E. Hvichia, L. J. Kricka and P. Wilding, Nucleic Acids Res., 1996, 24, 375 CrossRef CAS.
- J. Cheng, M. A. Shoffner, G. E. Hvichia, L. J. Kricka and P. Wilding, Nucleic Acids Res., 1996, 24, 380 CrossRef CAS.
- W. H. Grover, A. M. Skelley, C. N. Liu, E. T. Lagally and R. A. Mathies, Sens. Actuators B: Chem., 2003, 89, 315 CrossRef.
- M. A. Unger, H. P. Chou, T. Thorsen, A. Scherer and S. R. Quake, Science, 2000, 288, 113 CrossRef CAS.
- D. J. Beebe, J. S. Moore, J. M. Bauer, Q. Yu, R. H. Liu, C. Devadoss and B. H. Jo, Nature, 2000, 404, 588 CrossRef CAS.
- P. Woias, Sens. Actuators B: Chem., 2005, 105, 28 CrossRef.
- D. J. Laser and J. G. Santiago, J. Micromech. Microeng., 2004, 14, R35 CrossRef.
- S. Shoji and M. J. Esashi, J. Micromech. Microeng., 1994, 4, 157 CrossRef CAS.
- K. Handique, D. T. Burke, C. H. Mastrangelo and M. A. Burns, Anal. Chem., 2001, 73, 1831 CrossRef CAS.
- V. Namasivayam, R. G. Larson, D. T. Burke and M. A. Burns, J. Micromech. Microeng., 2003, 13, 261 CrossRef CAS.
- T. Vilkner, D. Janasek and A. Manz, Anal. Chem., 2004, 76, 3373 CrossRef CAS.
- V. Namasivayam, R. Lin, B. Johnson, S. Brahmasandra, Z. Razzacki, D. T. Burke and M. A. Burns, J. Micromech. Microeng., 2004, 14, 81 CrossRef CAS.
- K. B. Mogensen, H. Klank and J. P. Kutter, Electrophoresis, 2004, 25, 3498 CrossRef CAS.
- P. Ertl, C. A. Emrich, P. Singhal and R. A. Mathies, Anal. Chem., 2004, 76, 3749 CrossRef CAS.
- E. Thrush, O. Levi, L. J. Cook, J. Deich, A. Kurtz, S. J. Smith, W. E. Moerner and J. S. Harris, Sens. Actuators B: Chem., 2004, 105, 393.
- T. Kamei, B. M. Paegel, J. R. Scherer, A. M. Skelley, R. A. Street and R. A. Mathies, Anal. Chem., 2003, 75, 5300 CrossRef CAS.
- M. A. Burns, B. N. Johnson, S. N. Brahmasandra, K. Handique, J. R. Webster, M. Krishnan, T. S. Sammarco, F. P. Man, D. Jones, D. Heldsinger, V. Namasivayam, C. H. Mastrangelo and D. T. Burke, Science, 1998, 282, 484 CrossRef CAS.
- L. C. Waters, S. C. Jacobson, N. Kroutchinina, J. Khandurina, R. S. Foote and J. M. Ramsey, Anal. Chem., 1998, 70, 5172 CrossRef CAS.
- E. T. Lagally, C. A. Emrich and R. A. Mathies, Lab Chip, 2001, 1, 102 RSC.
- C. G. Koh, W. Tan, M. Q. Zhao, A. J. Ricco and Z. H. Fan, Anal. Chem., 2003, 75, 4591 CrossRef CAS.
- E. T. Lagally, J. R. Scherer, R. G. Blazej, N. M. Toriello, B. A. Diep, M. Ramchandani, G. F. Sensabaugh, L. W. Riley and R. A. Mathies, Anal. Chem., 2004, 76, 3162 CrossRef CAS.
- R. H. Liu, J. N. Yang, R. Lenigk, J. Bonanno and P. Grodzinski, Anal. Chem., 2004, 76, 1824 CrossRef CAS.
- For example, the high-throughput systems from Affymetrix, Applied Biosystems, Agilent, Nanogene, CombiMatrix, Orchid Biosciences and Nanostream, to name a few.
- These include the point-of-care systems from Sandia National Lab (μChemLab system), Cepheid (GeneXpert system), HandyLab and other companies.
- A. Gulliksen, L. A. Solli, K. S. Drese, O. Sorensen, F. Karlsen, H. Rogne, E. Hovig and R. Sirevag, Lab Chip, 2005, 5, 416 RSC.
- N. Christodoulides, S. Mohanty, C. S. Miller, M. C. Langub, P. N. Floriano, P. Dharshan, M. F. Ali, B. Bernard, D. Romanovicz, E. Anslyn, P. C. Fox and J. T. McDevitt, Lab Chip, 2005, 5, 261 RSC.
- T. Vestad, D. W. M. Marr and J. Oakey, Micromech. Microeng., 2004, 14, 1503 Search PubMed.
- V. Srinivasan, V. K. Pamula and R. B. Fair, Lab Chip, 2004, 4, 310 RSC.
- N. J. Cox and C. A. Bender, Semin. Virol., 1995, 6, 359 CrossRef CAS.
- R. Snacken, A. P. Kendal, L. R. Haahein and J. M. Woods, Emerging Infect. Dis., 1999, 5, 195 Search PubMed.
- M. R. Hilleman, Vaccine, 2002, 20, 3068–3087 CrossRef.
- R. Pal, M. Yang, B. N. Johnson, D. T. Burke and M. A. Burns, Anal. Chem., 2004, 76, 3740 CrossRef CAS.
- M. Yang, R. Pal and M. A. Burns, Micromech. Microeng., 2005, 15, 221 Search PubMed.
- S. N. Brahmasandra, V. M. Ugaz, D. T. Burke, C. H. Mastrangelo and M. A. Burns, Electrophoresis, 2001, 22, 300 CrossRef CAS.
- Manual of EDVO-Kit #334, EDVOTEK®, West Bethesda, MD.
- Y. Huang, E. L. Mather, J. L. Bell and M. Madou, Anal. Bioanal. Chem., 2002, 372, 49 CrossRef CAS.
- J. Lichtenberg, N. F. de Rooij and E. Verpoorte, Talanta, 2002, 56, 233 CrossRef CAS.
- R. C. Anderson, X. Su, G. J. Bogdan and J. Fenton, Nucleic Acids Res., 2000, 28, e60 CrossRef CAS.
- P. K. Yuen, L. J. Kricka, P. Fortina, N. J. Panaro, T. Sakazume and P. Wilding, Genome Res., 2001, 11, 405 CrossRef CAS.
- J. Khandurina, T. E. Mcknight, S. C. Jacobson, L. C. Waters, R. S. Foote and J. M. Ramsey, Anal. Chem., 2000, 72, 2995 CrossRef CAS.
- L. C. Waters, S. C. Jacobson, N. Kroutchinina, J. Khandurina, R. S. Foote and J. M. Ramsey, Anal. Chem., 1998, 70, 158 CrossRef CAS.
- J. Cheng, E. L. Sheldon, L. Wu, A. Uribe, L. O. Gerrue, J. Carrino, M. J. Heller and J. P. O'Connell, Nat. Biotechnol., 1998, 16, 541 CAS.
Footnote |
| † These authors contribute equally to the paper. |
| This journal is © The Royal Society of Chemistry 2005 |
