DOI:
10.1039/B503336E
(Paper)
J. Mater. Chem., 2005,
15, 3181-3186
Oxygen sensing materials based on mesoporous silica MCM-41 and Pt(II)–porphyrin complexes†
Received
8th March 2005
, Accepted 10th June 2005
First published on 27th June 2005
Abstract
The preparation and properties of luminescent oxygen sensing materials based on two Pt(II)–porphyrin complexes: platinum meso-tetrakis(4-N-methylpyridyl)porphyrin (PtTMPyP4+) and platinum meso-tetrakis(4-N-pyridyl)porphyrin (PtTPyP) assembled in mesoporous silica (MCM-41) are described. The luminescence of Pt(II)–porphyrin/MCM-41 assembly materials can be extremely quenched by molecular oxygen with good sensitivity (I0/I100 > 7) and rapid response times (<1 s) suggest that the Pt(II)–porphyrin/MCM-41 system can be used for developing oxygen sensors. PtTMPyP4+/MCM-41 (20 mg g−1) exhibits very high sensitivity (I0/I100 > 50). Even when the concentration of oxygen is 1%, the luminescence intensity of PtTMPyP4+/MCM-41 (20 mg g−1) can be quenched by 83.33%.
Introduction
Over the past decades, luminescence-based optical oxygen sensors have been greatly developed because the determination of molecular oxygen in both the gas and liquid phase is important in many different fields such as analytical chemistry, medical chemistry and environmental and industrial applications.1–4 These sensors are based upon the principle that oxygen is a powerful quencher of the luminescent intensity and lifetime of luminescent complexes, and the key factors that play a role include the optical properties of the luminescent complex and the solubility and diffusion coefficient of oxygen in the matrix. The most commonly used complexes for this application are transition metal complexes especially ruthenium(II) polypyridyl or phenanthroline complexes5–7 and metalloporphyrins8–10 owing to their high quantum yields, large Stokes’ shifts and long luminescent lifetimes. The host materials used to encapsulate the luminescent complexes are sol–gel and polymer films. Some interesting systems based on sol–gel or polymer immobilized transition metal complexes have been reported.6,10–13
Mesoporous materials have attracted considerable attention recently for their synthesis and functionalization.14–20 The highly controllable and monodisperse nature of the large accessible pore size, high surface area and periodic nano-scale pore spacing make mesoporous materials attractive media for applications such as heterogeneous catalytic, environmental, sensory or electronic media. Mesoporous materials are able to physically encapsulate and immobilize the functional molecules in the pores, while at the same time the existence of channels in mesoporous silica allows the transportation of solvent and other small molecules or ions into the interior of the mesoporous silica. As the luminescence of molecules incorporated in a mesoporous material could be quenched by molecules and gases surrounding the mesoporous material, it is possible to make mesoporous materials that have optical properties which are sensitive to the presence of target molecules, i.e. to make mesoporous chemical sensors.21–23 It is worth noting that the successful synthesis of transparent and hard mesoporous spheres opens an efficient avenue to the fabrication of new materials with useful optical properties.24–26
Recent work carried out by our group27,28 and others29 has demonstrated that [Ru(bpy)3]2+ can be incorporated into mesoporous silica. In this report, we present the preparation and properties of two oxygen sensing materials based on two metalloporphyrins, platinum meso-tetrakis(4-N-methylpyridyl)porphyrin (PtTMPyP4+) and platinum meso-tetrakis(4-N-pyridyl)porphyrin (PtTPyP) assembled in mesoporous silica (MCM-41).
Experimental
The free base meso-tetrakis(4-N-pyridyl)porphyrin (H2TPyP) was prepared using a variation of a method in the literature.30 H2TPyP (50 mg) was refluxed with 50 mg PtCl2 in 700 ml of anhydrous degassed benzonitrile solution in an inert atmosphere for more than 30 h until no free base was left as revealed by TLC. The mixture was cooled to room temperature and the solvent was removed by vacuum. The crude product was purified by column chromatography to obtain 43 mg pure PtTPyP. PtTMPyP4+ was synthesized according to the literature procedures.31 The synthesis scheme of Pt(II)–porphyrins applied in the study is given in the electronic supplementary information (ESI).†
Mesoporous silica (MCM-41) was prepared from cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), and NaOH according to the literature.32 Then the template was removed from the mesoporous silicate by calcination at 560 °C for 8 h.
The Pt(II)–porphyrin/MCM-41 assembly materials were prepared by the following procedure. MCM-41 was added into the aqueous solution of PtTMPyP4+ or chloroform solution of PtTPyP. After stirring, filtration, washing with the corresponding solution, and drying in air, PtTMPyP4+/MCM-41 or PtTPyP/MCM-41 was obtained. For example, 1 mg PtTMPyP4+ was dissolved in 8 ml water to form a red transparent aqueous solution, then 50 mg MCM-41 was added and the mixture was stirred for 1 h. The mixture was filtered to give a purplish powder. The purplish powder was washed repeatedly with water until the filtered water was colorless, and after drying in air 20 mg PtTMPyP4+/g MCM-41 was obtained. Samples with different loading levels of PtTMPyP4+ (10, 20 and 40 mg PtTMPyP4+/g MCM-41) or PtTPyP (20, 40 and 80 mg PtTPyP/g MCM-41) were prepared by changing the concentration of the starting solution of Pt(II)–porphyrin. UV-vis spectrophotometric analysis of the filtrate showed the quantity of remaining PtTPyP or PtTMPyP4+, which was unincorporated into MCM-41.27,28 The Pt(II)–porphyrin contents of PtTPyP/MCM-41 and PtTMPyP4+/MCM-41 samples are listed in Table 1.
Table 1 Pt(II)–porphyrin contents of PtTPyP/MCM-41and PtTMPyP4+/MCM-41
| |
(PtTPyP/MCM-41)/mg g−1 |
(PtTMPyP4+/MCM-41)/mg g−1 |
| 20 |
40 |
80 |
10 |
20 |
40 |
| Pt(II)–porphyrin content (%) |
2.0 |
4.0 |
7.3 |
1.0 |
2.0 |
4.0 |
Each Pt(II)–porphyrin/MCM-41 sample was fixed inside a laboratory-made cell that was equipped with two quartz windows and an air-tight stopper which had inlet and outlet lines to allow gas flow. To investigate the sensing properties of Pt(II)–porphyrins to different oxygen concentrations, dry nitrogen and dry oxygen were mixed to obtain gas mixtures. All luminescence measurements were performed in the dark.
The powder X-ray diffraction (XRD) patterns were recorded on a Siemens D5005 diffractometer with Cu-Kα radiation, step size 0.02° and step count 1 s. UV-vis absorption spectra were obtained using a Variancary 500 UV-vis spectrophotometer. The luminescence spectra and response curves were obtained using a PTI time-resolved emission spectrophotometer.
Results and discussion
In order to examine whether Pt(II)–porphyrin molecules were incorporated inside the channels of MCM-41 or only absorbed by the interior surface of MCM-41, uncalcined MCM-41 was put into the Pt(II)–porphyrin solution to give a non-luminescent product. However, calcined MCM-41, which has unoccupied channels, in Pt(II)–porphyrin solution resulted in the formation of a red luminescent solid under excitation by UV light (λexc = 404 nm). The luminescence of the solid remained unchanged even after the solid was washed repeatedly with solvent. It is demonstrated that the Pt(II)–porphyrin molecules are incorporated inside the channels of MCM-41. The powder X-ray diffraction (XRD) spectra (in Fig. 1) of PtTMPyP4+/MCM-41 show identical patterns with MCM-41. This indicates that after the incorporation of Pt(II)–porphyrin, the hexagonal arrangement of channels in MCM-41 remains unchanged. The UV-vis absorption spectruma of PtTMPyP4+/MCM-41 shows a similar profile to that of PtTMPyP4+ in water (Fig. 2). The peak at 404 nm is due to the Soret band of PtTMPyP4+, and peaks due to the Q bands are at 512 nm and 546 nm. The above experiments indicate that after the Pt(II)–porphyrin is incorporated into MCM-41, the structure of MCM-41 is not destroyed and Pt(II)–porphyrin/MCM-41 exhibits the optical properties of the Pt(II)–porphyrin.
 |
| | Fig. 1 Powder X-ray spectra of MCM-41 (a) and PtTMPyP4+/MCM-41 (b). Insert: the molecular structure of PtTMPyP4+. | |
The emission spectra recorded for PtTPyP/MCM-41 and PtTMPyP4+/MCM-41 with different loading levels are presented in Figs. 3 and 4, respectively. For PtTPyP/MCM-41, the emission spectra exhibit a red shift from 640 nm to 645 nm and peak broadening with increasing porphyrin loading level. A similar tendency was observed for the PtTMPyP4+/MCM-41 system. The molecular arrangement and aggregated forms should vary with the increase of loading level of PtTPyP or PtTMPyP4+, which may lead to the emission red shift and peak broadening.33,34 The other possible explanation is that the increase of Pt(II)–porphyrin concentration can enhance the intermolecular interactions between Pt(II)–porphyrin molecules in the channels. This may lead to the formation of excimers between adjacent lumophores in the channels that results in the red shift and peak broadening of the emission spectra.27,28
 |
| | Fig. 3 Emission spectra of PtTPyP/MCM-41: 20 mg g−1 (a), 40 mg g−1 (b) and 80 mg g−1 (c). Insert: the molecular structure of PtTPyP. | |
 |
| | Fig. 4 Emission spectra of PtTMPyP4+/MCM-41: 10 mg g−1 (a), 20 mg g−1 (b) and 40 mg g−1 (c). | |
The luminescence of most Pt(II)–porphyrin complexes could be quenched effectively by molecular oxygen. The mechanism of the quenching process consists of exchange energy transfer from the lowest triplet excited state of metalloporphyrin to molecular oxygen, which is accompanied by the formation of singlet oxygen. The room temperature emission spectra, which are recorded for PtTPyP/MCM-41 (20 mg g−1) under different concentrations of oxygen, are presented in Fig. 5. The emission peak of PtTPyP/MCM-41 (20 mg g−1) is at 640 nm, and constant under different oxygen concentrations. However, the relative intensity decreased markedly with increasing oxygen concentration. The variation of the emission spectra of PtTPyP/MCM-41 (40 mg g−1) displays similar trends to that of PtTPyP/MCM-41 (20 mg g−1). The relative luminescent intensities of PtTPyP/MCM-41 (20 mg g−1) and PtTPyP/MCM-41 (40 mg g−1) decrease by 86.4% and 85.7%, respectively, upon changing from pure nitrogen to pure oxygen conditions. The oxygen concentration dependent emission spectra of PtTMPyP4+/MCM-41 (20 mg g−1) are presented in Fig. 6. Upon increasing oxygen concentration, the emission peak of the sample remains at 668 nm and the emission intensity drops quickly. For PtTMPyP4+/MCM-41 (40 mg g−1) a similar result was obtained. The relative luminescent intensities of PtTMPyP4+/MCM-41 (20 mg g−1) and PtTMPyP4+/MCM-41 (40 mg g−1) decrease by 98.2% and 95.6%, respectively, upon changing from pure nitrogen to pure oxygen conditions. The PtTMPyP4+/MCM-41 system is more sensitive compared with the PtTPyP/MCM-41 system. This can be attributed to the fact that the excited state lifetime of PtTMPyP4+ is longer than that of PtTPyP and the quenching sensitivity increases with the unquenched lifetime of the luminescent complex.23,35,36 On the other hand, the distributed and aggregated forms of PtTPyP and PtTMPyP4+ in MCM-41 should be different, which can also influence the sensitivity.37
 |
| | Fig. 5 Emission spectra of PtTPyP/MCM-41 (20 mg g−1) under different oxygen concentrations. | |
 |
| | Fig. 6 Emission spectra of PtTMPyP4+/MCM-41 (20 mg g−1) under different oxygen concentrations. | |
Figs. 7 and 8 present the Stern–Volmer plots for PtTPyP/MCM-41 (20 mg g−1) and PtTMPyP4+/MCM-41 (20 mg g−1), respectively. The plots are nonlinear within a wide range of oxygen concentration. It has been demonstrated that for heterogeneous systems, Stern–Volmer plots for sensor systems based on luminescence quenching often display non-linear features within a wide range of oxygen concentration,38–41 which is attributed to the simultaneous presence of static and dynamic quenching, and the inequality of the microenvironment of the immobilized lumophore molecules in the matrix.42,43 Close investigation reveals that the Stern–Volmer plots exhibit perfect linear characteristics when the concentration of oxygen varied from 0 to 5% (insets of Figs. 7 and 8). The plots indicate that high sensitivity is obtained in the oxygen concentration range of 0–5%. Even when the concentration of oxygen is only 1%, the quenching of PtTMPyP4+/MCM-41 and PtTPyP/MCM-41 can reach 83.33% (I0/I = 6) and 33.33% (I0/I = 1.33), respectively. It is well know that the measurement of oxygen at low concentration is more important, so Pt(II)–porphyrin/MCM-41 has great potential for application in oxygen sensors.
 |
| | Fig. 7 Stern–Volmer plot for PtTPyP/MCM-41 (20 mg g−1) at different concentrations of oxygen. (I0 and I are the luminescent intensities in the absence and in the presence of oxygen). Inset: Stern–Volmer plot at low oxygen concentration. | |
 |
| | Fig. 8 Stern–Volmer plot for PtTMPyP4+/MCM-41 (20 mg g−1) at different concentrations of oxygen. Inset: Stern–Volmer plot at low oxygen concentration. | |
For oxygen sensors the response time is also very important. Generally, 95% response time, i.e., t↓(95%, N2
→ O2), is defined as the time required for the luminescent intensity to decrease by 95% on changing from 100% nitrogen to 100% oxygen. Similarly, 95% recovery time, i.e., t↑(95%, O2
→ N2), means the time required for the luminescent intensity to reach the 95% of the initial value recorded under 100% nitrogen on changing from 100% oxygen to 100% nitrogen. Figs. 9 and 10 show the response properties of PtTPyP/MCM-41 (20 mg g−1) and PtTMPyP4+/MCM-41 (20 mg g−1), respectively. Upon increasing oxygen concentration, the emission intensity drops very quickly, while upon decreasing oxygen concentration, the emission intensity increases and recovers to the initial level under 100% nitrogen again. This cycle is repeated and the emission intensity changes are monitored when the sample is exposed to an alternating atmosphere of nitrogen and oxygen, indicating that the emission intensity changes are reversible. The values of response time (t↓), recovery time (t↑) and sensitivity (I0/I100, where I0 and I100 represent the luminescent intensities in 100% nitrogen and 100% oxygen, respectively) are listed in Table 2. All oxygen sensing materials based on Pt–porphyrins and MCM-41 have short response times (<1 s) and good sensitivity (I0/I100 > 7), especially PtTMPyP4+/MCM-41 (20 mg g−1) with a very fast response time (<0.5 s), recovery time (<30 s) and excellent sensitivity (I0/I100 > 50). The oxygen sensing property of this kind of material is much better than that of other Pt–porphyrin based sensing materials reported previously.20–23 The results show that the recovery time is significantly longer than the response time, similar to results obtained for Pt-OEP in polymer medium.44 Because small molecules such as oxygen are well known to be adsorbed strongly on silica surfaces, the longer recovery time can be attributed to slow desorption of oxygen from the silica surface in the support.45 PtTMPyP4+/MCM-41 and PtTPyP/MCM-41 show similar response times, but their recovery times are obviously different. This phenomenon may be because the O2 desorption rate of PtTMPyP4+/MCM-41 is faster than that of PtTPyP/MCM-41.
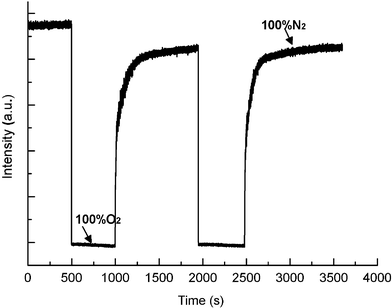 |
| | Fig. 9 Response time and relative intensity change of PtTPyP/MCM-41 (20 mg g−1) to alternate environments of 100% nitrogen and 100% oxygen. | |
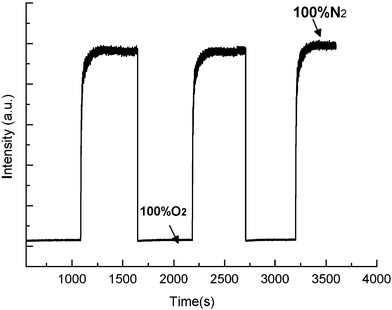 |
| | Fig. 10 Response time and relative intensity change of PtTMPyP4+/MCM-41 (20 mg g−1) to alternate environments of 100% nitrogen and 100% oxygen. | |
Table 2 Oxygen sensing properties of PtTPyP/MCM-41and PtTMPyP4+/MCM-41
| |
(PtTPyP/MCM-41)/mg g−1 |
(PtTMPyP4+/MCM-41)/mg g−1 |
| 20 |
40 |
20 |
40 |
|
I
0/I100 |
7.34 |
7.00 |
55.50 |
23.53 |
|
t↓/s |
0.75 |
0.50 |
0.33 |
0.36 |
|
t↑/s |
204.00 |
220.12 |
26.62 |
29.58 |
Conclusions
New oxygen sensing materials based on Pt(II)–porphyrin assembled in MCM-41 were prepared. Oxygen sensing studies demonstrate that the luminescence of the materials exhibits strongly oxygen concentration dependent characteristics and is easily quenched by oxygen. The oxygen sensing property of PtTMPyP4+/MCM-41 is obviously better than that of PtTPyP/MCM-41. The reasons for these results remain unclear and highlight the fact that PtTMPyP4+/MCM-41 system is a good candidate for developing high performance oxygen sensing materials. In this paper our interests are focused on the functions of PtTPyP/MCM-41 and PtTMPyP4+/MCM-41; investigation of the detailed photophysics and operation mechanism of Pt(II)–porphyrin/MCM-41 and further optimization of the Pt–porphyrin/mesoporous silica system by using other mesoporous materials such as MCM-48 are under way.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, the Major State Basic Research Development Program (2002CB613401) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT0422).
References
- Y. Amao, T. Miyashita and I. Okura, Analyst, 2000, 125, 871 RSC.
- E. Singer, G. L. Duveneck, M. Ehrat and H. M. Widmer, Sens. Actuators A, 1994, 542, 41.
- M. A. Chan, S. K. Lam and D. Lo, J. Fluoresc., 2002, 12, 327 CrossRef CAS.
- F. G. Gao, A. S. Jeevarajan and M. M. Anderson, Biotechnol. Bioeng., 2004, 86, 425 CrossRef CAS.
- M. T. Murtagh and M. R. Shahriari, Chem. Mater., 1998, 10, 3862 CrossRef CAS.
- P. Payra and P. K. Dutta, Microporous Mesoporous Mater., 2003, 64, 109 CrossRef CAS.
- E. Yavin, L. Weiner, R. A. Yellin and A. Shanzer, J. Phys. Chem. A, 2004, 108, 9274 CrossRef CAS.
- D. B. Papkovsky, G. V. Ponomarev, W. Trettnak and P. O'Leary, Anal. Chem., 1995, 67, 4112 CrossRef CAS.
- Y. Amao, K. Asai and I. Okura, J. Porphyrins Phthalocyanines, 2000, 4, 179 CrossRef CAS.
- R. N. Gillanders, M. C. Tedford, P. J. Crilly and R. T. Bailey, Anal. Chim. Acta, 2004, 502, 1 CrossRef CAS.
- S. -K. Lee and I. Okura, Analyst, 1997, 122, 81 RSC.
- S. -K. Lee and I. Okura, Anal. Commun., 1997, 34, 185 RSC.
- S. M. Borisov and V. V. Vasil'ev, J. Anal. Chem., 2004, 59, 155 CrossRef CAS.
- A. M. Liu, K. Hidajat, S. Kawi and D. Y. Zhao, Chem. Commun., 2000, 1145 RSC.
- X. Feng, G. E. Fryxell, L. Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923 CrossRef CAS.
- Z. Luan, J. Y. Bae and L. Kevan, Chem. Mater., 2000, 12, 3202 CrossRef CAS.
- M. Kaneda, T. Tsubakiyama, A. Carlsson, Y. Sakamoto, T. Ohsuna and O. Terasaki, J. Phys. Chem. B, 2002, 106, 1256 CrossRef CAS.
- M. H. Lim, C. F. Blanford and A. Stein, J. Am. Chem. Soc., 1997, 119, 4090 CrossRef CAS.
- I. Honma and H. S. Zhou, Adv. Mater., 1998, 10, 1532 CrossRef CAS.
- Y. Ueno, A. Tate, O. Niwa, H. S. Zhou, T. Yamada and I. Honma, Chem. Commun., 2004, 746 RSC.
- H. Fau, Y. Lu, A. Stump, S. T. Reed, T. Baer, R. Schunk, V. Perezlund, G. P. Lopez and C. J. Brinker, Nature, 2000, 405, 56 CrossRef.
- G. Wirnsberger, B. J. Scott and G. D. Stucky, Chem. Commun., 2001, 119 RSC.
- A. B. Descalzo, D. Jimenez, M. D. Marcos, R. M-Máñez, J. Soto, J. E. Haskouri, C. Guillém, D. Beltrán, P. Amorós and M. V. Borrachero, Adv. Mater., 2002, 14, 966 CrossRef CAS.
- Q. Huo, J. Feng, F. Schüth and G. D. Stucky, Chem. Mater., 1997, 9, 14 CrossRef CAS.
- M. Grün, I. Lauer and K. K. Unger, Adv. Mater., 1997, 9, 254 CrossRef.
- S. Han and T. Hyeon, Chem. Commun., 1999, 1955 RSC.
- M. Fang, Y. Wang, P. Zhang, S. G. Li and R. R. Xu, J. Luminesc., 2000, 91, 67 CrossRef CAS.
- P. Zhang, J. H. Guo, Y. Wang and W. Q. Pang, Mater. Lett., 2002, 53, 400 CrossRef CAS.
- M. Ogawa, T. Nakamura, J. I. Mori and K. Kuroda, J. Phys. Chem., 2000, 104, 8554 Search PubMed.
- F. R. Longo, M. G. Finarelli and J. B. Kim, Heterocycl. Chem., 1969, 6, 927 CrossRef CAS.
- R. F. Pasternack, P. R. Huber, P. Boyd and G. Engasser, J. Am. Chem. Soc., 1972, 4511 CrossRef CAS.
- B. Onida, B. Bonelli, L. Flora, F. Geobaldo, C. O. Arean and E. Garrone, Chem. Commun., 2001, 2216 RSC.
- K. Kano, K. Fukuda, H. Wakami, R. Nishiyabu and R. F. Pasternack, J. Am. Chem. Soc., 2000, 122, 7494 CrossRef CAS.
- Y. H. Kim, D. H. Jeong, D. Kim, S. C. Jeoung, H. S. Cho, S. K. Kim, N. Aratani and A. Osuka, J. Am. Chem. Soc., 2002, 123, 76.
- V. V. Vasil'ev and S. M. Borisov, Sens. Actuators B., 2002, 82, 272 CrossRef.
- B. H. Han, I. Manners and M. A. Winnik, Chem. Mater., 2005 Search PubMed , in press.
- I. Klimant and O. S. Wolfbeis, Anal. Chem., 1995, 67, 3160 CrossRef CAS.
- B. D. MacCraith, C. M. McDonagh, G. O'Keeffe, E. T. Keyes, J. G. Vos, B. O'Kelly and J. F. McGilp, Analyst, 1993, 118, 385 RSC.
- W. Xu, R. Schmidt, M. Whaley, J. N. Demas, B. A. DeGraff, E. K. Karikari and B. L. Farmer, Anal. Chem., 1995, 67, 3172 CrossRef CAS.
- E. R. Carraway, J. N. Demas, B. A. DeGraff and J. R. Bacon, Anal. Chem., 1991, 63, 337 CrossRef CAS.
-
J. R. Lakowicz, Principle of Fluorescence Spectroscopy, Plenum, New York, 1986, p. 266 Search PubMed.
- P. Hartmann, M. J. P. Leiner and M. E. Lippitsch, Anal. Chem., 1995, 67, 88 CrossRef CAS.
- M. E. Lippitsch and S. Draxler, Sens. Actuators B, 1993, 11, 97 CrossRef.
- A. Mills and A. Lepre, Anal. Chem., 1997, 69, 4653 CrossRef CAS.
- A. Yekta, Z. Masoumi and M. A. Winnik, Can. J. Chem., 1995, 73, 202.
|
| This journal is © The Royal Society of Chemistry 2005 |
Click here to see how this site uses Cookies. View our privacy policy here. 









