Influence of surfactant assembly on the formation of calcium phosphate materials—A model for dental enamel formation
Christabel E.
Fowler
a,
Mei
Li
b,
Stephen
Mann
b and
Henry C.
Margolis
*c
aGlaxoSmithKline, St Georges Avenue, Weybridge, Surrey, UK KT13 0DE
bSchool of Chemistry, University of Bristol, Bristol, UK BS8 1TS
cDepartment of Biomineralization, The Forsyth Institute, 140 The Fenway, Boston, Massachusetts 02115, USA. E-mail: hmargolis@forsyth.org
First published on 7th July 2005
Abstract
Novel calcium phosphate materials were synthesized from solutions containing the surfactant bis(2-ethylhexyl)sulfosuccinate sodium salt (AOT), water and oil. A range of morphologies was obtained by varying the relative concentrations of the solution components. A material with structural features resembling tooth enamel was produced from a highly viscous reaction solution. This material consisted of bundles of co-aligned filaments, 750 nm–1 µm in length and 250–350 nm wide. Each bundle contained 10–20 filaments, identified as hydroxyapatite crystals. Electron diffraction of the bundles resulted in an arc pattern indicative of elongated aligned crystals, and similar to that known for dental enamel. Systems such as this may be used as models to gain insight into the mechanisms involved in the biomineralization of tooth enamel. Importantly, we have provided new evidence in support of the hypothesis that hierarchical structures in nature result from cooperative interactions between organic assembly and crystal growth. Amorphous calcium phosphate nanoparticles, hollow spheres, spherical octacalcium phosphate aggregates of plates and elongated plates of calcium hydrogen phosphate dihydrate were also obtained under different sets of conditions that altered AOT assembly and solution viscosity. These latter findings illustrate further the significant influence of organic assembly on the formation of calcium phosphate materials.
Introduction
Calcium phosphates are among the most widely utilized minerals in living organisms. The human body, for example, employs calcium phosphates as the inorganic component of bones and teeth. These tissues, like a variety of other inorganic/biomolecule composites found in nature, have unique structures and properties that are tailored to carry out specific functions.Unlike the other mineralized tissues of the body (i.e., bone, dentin and cementum), mature dental enamel is almost fully mineralized, having a mineral content greater than 95%, making it the hardest tissue in the human body. The mineral phase closely resembles hydroxyapatite, but is enriched with carbonate, sodium, and magnesium. The enamel structure is also uniquely typified by highly elongated crystals organized into rods or bundles, interspaced with inter-rod material (mainly small amounts of proteins, lipids and randomly oriented crystals).1 Interwoven patterns of these rods are aligned along directions of stress, providing the tissue vital strength.
Although much is now known about the overall process of dental enamel formation,2 relatively little is known about the precise mechanism by which the highly organized enamel structure is produced in vivo. Like other mineralized tissues, dental enamel is the product of specialized cells (ameloblasts, in this case), and protein-mediated biomineralization which takes place within an extracellular matrix. Amelogenin, a 20 kDa protein unique to dental enamel, is the major component of this matrix,3 and has been shown to be essential for proper enamel mineralization in vivo.4 Amelogenin is predominantly hydrophobic, with a hydrophilic C-terminus of 13 amino acids. All proteins are generally considered to be amphiphilic biopolymers. However, the charge separation in amelogenin is considerably better defined and closer to that of traditional surfactants when compared with other proteins. Recent studies have suggested that self-assembly of amelogenin to form “nanospheres” may be important in the process of enamel formation.5–9 It has been proposed that these nanospheres may produce higher order structures2,10 which regulate the enamel mineral organization, however, these structures and the mechanism of their formation have not been elucidated.
Organized assemblies of amphiphilic molecules have been extensively shown to provide a suitable environment for the controlled nanoscale synthesis of biologically relevant inorganic materials (e.g., calcium phosphates,11–13 calcium carbonates,14,15 silicates,16,17 iron oxides18,19). Such systems have been investigated due to their relevance to the field of biomineralization, but also as potential routes to the synthesis of organized inorganic materials.20 A recent report described the preparation of a synthetic amphiphilic peptide, and its subsequent mineralization to produce a composite with structural features in common with mineralized collagen fibrils in bone.21
Since the full-length amelogenin molecule (i.e., with the hydrophilic C-terminus intact) has structural similarities to synthetic amphiphilic molecules, model systems employing such molecules may provide insight into the mechanism of enamel mineral formation. Mechanistic details of approaches to produce hybrid nanostructures, and their relevance to biomineralization processes, have been described in detail in a recent review.22 In addition to providing mechanistic information, enamel model systems may lead to the production of calcium phosphates and other materials with controlled compositional, dimensional, and morphological specificity. Such materials hold potential as biomedical implants in dentistry and orthopedics, controlled release devices for drug delivery and chromatographic supports, for example. Of direct relevance, a number of studies have focused on the controlled synthesis of calcium phosphate materials possessing high aspect ratios.23–25
The ability of surfactants to affect crystallization processes, as well as mineral growth, is well recognized. Promotion and inhibition of nucleation, crystal growth, and aggregation have all been studied.26–30 As a result of interactions between the surfactant head group and inorganic ions/crystal surfaces, controllable to some extent by their degree of hydration, the dominant crystal phase under specific conditions can be altered, as well as the material's morphological features. Interestingly, the ability of amelogenin to interact with crystal surfaces and affect crystal growth31,32 is markedly affected when the surfactant-like character of amelogenin is altered by the removal of its hydrophilic C-terminus (head group).
The aim of this investigation was to synthesize calcium phosphate materials with enamel-like structural features, but under the control of better-defined synthetic organic structures. A consideration of the role of the organic agent may provide insight into the mechanisms by which proteins and other biomolecules affect the growth and organization of calcium phosphates in the formation of dental enamel. Sodium AOT (bis(2-ethylhexyl)sulfosuccinate) was chosen as the structure-directing agent. This surfactant readily forms a number of well defined higher-order structures/phases in solution, and has previously been shown to interact strongly with calcium phosphates,33,34 carbonates,35 oxalates,27 sulfates36 and barium chromates,37 thereby affecting the growth pattern of these materials. Uniquely, the present studies were carried out within viscous reaction media, which likely resemble the conditions within which enamel crystals are formed in vivo. Although the nucleation and growth patterning of calcium phosphate materials has previously been shown to be influenced by the presence of surfactants and polymers, to the best of our knowledge, such studies have not resulted in the synthesis of bundles of elongated and well-aligned crystals of hydroxyapatite. Elongated crystals of hydroxyapatite produced have all been discrete.23–25
Experimental
Calcium phosphates were grown by mixing aqueous solutions of calcium chloride and potassium dihydrogen phosphate under ambient conditions in the presence of varying concentrations of the sodium salt of AOT (bis(2-ethylhexyl)sulfosuccinate) (Fluka Chemika, Buchs, Switzerland), which was dissolved in mixtures of m-xylene (99% anhydrous, Aldrich Chemical Co., Milwaukee, WI, USA) and isooctane (99.7+% HPLC grade, Aldrich Chemical Co., Milwaukee, WI, USA) solvents. Stock solutions of calcium chloride dihydrate (0.15 M) and potassium dihydrogen phosphate (0.1 M) were prepared from reagent grade chemicals using distilled deionized water. The ratios of the solution components were varied in a series of experiments, and the products of the reactions analyzed. The molar ratios (with respect to calcium) of the reaction constituents used to create the various products are given in Table 1, along with w values which represent the molar ratio of water to AOT (w = [H2O]/[AOT]). As indicated in Table 1, under all experimental conditions, the calcium: phosphate ratio remained constant and equal to 1.5. Further experimental details are described in the following paragraphs.| Molar ratiosa | ||||||
|---|---|---|---|---|---|---|
| Final product | w Valueb | CaCl2 | KH2PO4 | AOT | Isooctane | m-Xylene |
| a Molar ratios are expressed relative to calcium content. Noted values represent actual amounts used, taking into consideration volume changes that occur in the preparation of AOT stock solutions. b w = [H2O]/[AOT]. | ||||||
| Bundles of filaments | 1.4 | 1.0 | 0.67 | 520 | 800 | 40 |
| Filamentous networks | 1.6 | 1.0 | 0.67 | 450 | 800 | 130 |
| Nanoparticles | 2.4 | 1.0 | 0.67 | 300 | 800 | 40 |
| Elongated narrow plates | 6.9 | 1.0 | 0.67 | 105 | 800 | — |
| Hollow spheres | 16.2 | 1.0 | 0.67 | 45 | — | 5000 |
Preparation of filament bundles
In a typical preparation, a stock solution of AOT was prepared by dissolving 3.5 g of the surfactant in a mixture of isooctane and m-xylene (2 ml isooctane + 75 µl m-xylene). Twenty (20) µl of the calcium stock solution was then added to 0.5 ml of the AOT solution, and 20 µl of potassium dihydrogen phosphate to another 0.5 ml aliquot of the AOT solution. These two solutions were vortexed separately at 800 rpm for 30 seconds. After being left to stand at room temperature for 60 minutes, the phosphate solution was added to the cloudy calcium solution and the highly viscous mixture was vortexed again for 30 seconds before aging at room temperature. After 72 hours, the reaction solution was no longer cloudy, but a faint white precipitate could be seen within the viscous matrix. Samples were removed and analyzed at selected times, as described below.Influence of reaction conditions
Table 1 summarizes the molar ratios of the reaction components used for subsequent experiments and the resulting products. Control materials were synthesized for each experiment, but without the inclusion of the AOT surfactant.Analyses
Samples were examined by transmission electron microscopy (TEM) using a JEOL 1200 EX microscope operating at 100 KeV. Samples for examination were prepared by re-suspending a small quantity of the reaction solution in ethanol, and allowing it to air dry on a carbon coated copper TEM grid. The grid was then passed through a droplet of isooctane to remove excess surfactant. Energy Dispersive X-ray Analysis (EDX) was carried out using an Oxford Instruments X-ray analysis ISIS300 system with a silicon detector and beryllium window.Dynamic light scattering (DLS) measurements were performed using a DynaPro-MS/X instrument to assess particle sizes in the initial solutions, and during subsequent reactions. Small angle X-ray scattering (SAXS) measurements were also undertaken to obtain particle size data for appropriate systems. SAXS experiments were completed at the University of Leoben, Austria. Samples for SAXS (Cu Kα radiation) were injected with a syringe into 0.5 mm diameter, 0.01 mm thick borosilicate capillary tubes, and examined over a scattering vector (Q) range of 0.2–9 nm−1.
Results
Formation of filament bundles
TEM analysis of a material prepared using the reverse micelle region of the AOT/isooctane/water phase diagram38 as a guide (w = 1.4), but with the addition of a small quantity of m-xylene, revealed bundles of aligned filaments (Fig. 1a). The filaments were found to be hydroxyapatite by electron diffraction. Each bundle contained 10 to 20 filaments. Bundle lengths ranged from 750 nm–1 µm, and widths from 250–350 nm. A detailed examination of the background of the grid revealed a large number of smaller individual threads, highly uniform and approximately 150 nm in length and 10 nm wide. No other particle morphologies were observed. Electron diffraction analysis of the bundles resulted in an arc-like pattern (Fig. 1b) similar to that seen in electron diffraction patterns from enamel rods.39 This pattern arises from the parallel alignment and packing of elongated crystals. A control sample that lacked AOT resulted in randomly orientated individual plate-like crystals of hydroxyapatite (Fig. 1c). These crystals were approximately 600 nm in length and 100 nm wide, exhibiting a much lower aspect ratio than the crystals forming the bundles above. | ||
| Fig. 1 (a) Bundles of aligned filaments of hydroxyapatite. Bundles were prepared under conditions of high AOT and low water concentration (w = 1.4), with a small quantity of m-xylene co-solvent. (b). Electron diffraction pattern of bundles of aligned hydroxyapatite crystals from (a) (arrow highlights arc pattern). A similar arc pattern is seen in electron diffraction patterns from enamel rods. (c). Control hydroxyapatite crystals prepared without AOT. Crystals are randomly orientated and not arranged in bundles. | ||
The bundles of crystals described above were evident 3 days after mixing the reagents. No further changes were observed for up to 12 months. However, aliquots taken after 1 and 2 days provided insight into the development of the bundles of fibres. After one day, TEM images showed a large number of low contrast spherical structures, 350–500 nm in diameter (Fig. 2a). No other structures were present at this time.
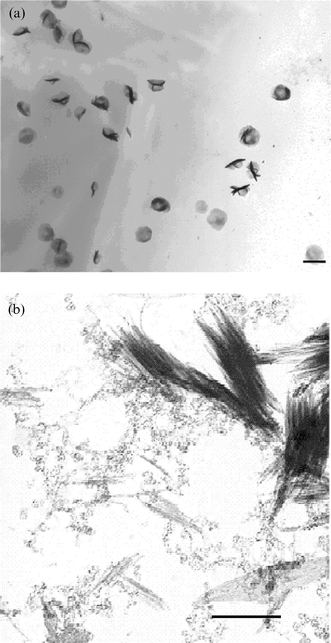 | ||
| Fig. 2 (a) Low contrast spherical structures detected during early stages of calcium phosphate bundle formation (after 24 h of reaction), scale bar = 500 nm. (b) Aggregated 5–70 nm particles, and bundles of filaments (scale bar = 200 nm). The beam sensitive nanoparticles were apparent after 48 h of reaction. | ||
Twenty-four (24) hours later, the low contrast spherical structures were still present, but in smaller numbers. Inspection of the background showed (Fig. 2b) numerous small beam-sensitive particles. These particles ranged from approximately 5–70 nm, and were often aggregated. A further abundant species comprised bundles of filaments similar to those observed after 3 days (Fig. 2b). High magnification images of the filaments showed them to be unstable, and electron diffraction confirmed their amorphous nature. The filaments appeared to be associated with the spheres on occasion, but this is likely to be a drying artefact. A small number of isolated bundles of filaments were also evident after 2 days incubation. These bundles again consisted of amorphous filaments. A final less abundant entity in this sample was larger spherical objects (not shown), 500–800 nm in diameter. These structures appeared to be composed of thin plates. The plates were 100–300 nm in length and 40–80 nm in width, and their sizes were uniform within a particular sphere. Similar structures to this have been noted previously in an investigation into the effect of anionic surfactants on the growth of calcium hydrogen phosphate dihydrate. These structures were identified as octacalcium phosphate.33,34
After 3 days of reaction, all spherical particles seen in earlier samples had disappeared. The only structures present at this time were the bundles of filaments and smaller individual filaments, consisting of crystalline hydroxyapatite (Fig. 1a).
SAXS scattering measurements were performed on the initial viscous calcium and phosphate solutions used to prepare the filament bundles. Both samples gave rise to patterns containing one relatively broad peak. Mean particle diameters were 2.6 nm for the calcium chloride sample, and 3.1 nm for the potassium dihydrogen phosphate sample (Table 2). These diameters suggest that the solutions may contain reverse micelles, consistent with predictions made from the AOT/water/isooctane ternary phase diagram.38
| Mineral phase | Final morphology | Initial mean particle size/nm | |
|---|---|---|---|
| CaCl2 solution | KH2PO4 solution | ||
| a Values were determined using DLS or where indicated (*) by SAXS. | |||
| Hydroxyapatite | Bundles of filaments | 2.6* | 3.1* |
| Hydroxyapatite | Filamentous networks | — | — |
| Amorphous CaP | Nanoparticles, 20 nm | 3.14 | 5.23 |
| Calcium hydrogen phosphate dihydrate | Elongated narrow plates | 5.12 | 6.44 |
| Amorphous CaP | Hollow spheres, 40–125 nm | 6.04 | 7.34 |
Influence of reaction conditions
From viscous mixtures containing a slightly lower molar ratio of AOT (w = 1.6), and a greater molar ratio of m-xylene (Table 1), extended filamentous networks were obtained (Fig. 3) at 72 h. High-resolution TEM images showed that branches of the networks consisted of interconnecting bundles of needle-like crystals. The bundles were 40–100 nm in width and contained 6–10 crystals that were co-aligned in a fashion similar to that seen in Fig. 1a. The open framework structure provided the material with a wide range of pore diameters (maximum diameter approximately 1 µm). Electron diffraction analysis confirmed that the networks consisted of hydroxyapatite. | ||
| Fig. 3 Extended networks of hydroxyapatite, scale bar = 1 µm. The networks were synthesised under conditions of slightly higher xylene and water content (w = 1.6) than the bundles of filaments. | ||
By increasing the w value (w = 2.4), while still aiming to work within the reverse micelle/microemulsion region of the AOT/isoocatane/water phase diagram, nanoparticles of amorphous calcium phosphate, approximately 20 nm in diameter, were produced (Fig. 4). The particles were present 72 h after the calcium and phosphate solutions were mixed, and were the only product under these conditions. DLS experiments were undertaken in order to characterize the initial reactant solutions giving rise to this product (Table 2). Mean diameters for the calcium and phosphate solutions were 3.14 nm (s.d. 0.10) and 5.23 nm (s.d. 0.08) respectively. A mean diameter of 5.20 nm (s.d. 0.10) was obtained when the ion solution was substituted by distilled water, again suggesting that the initial calcium solution contained reverse micelles and the phosphate solution a microemulsion.
 | ||
| Fig. 4 Nanoparticles of amorphous calcium phosphate, observed after 3 days of a reaction where w = 2.4. | ||
A product consisting exclusively of elongated narrow plates was obtained under conditions where w = 6.9 and no m-xylene solvent was present. Electron diffraction confirmed that the plates were calcium hydrogen phosphate dihydrate. This product was evident 72 h after mixing the calcium and phosphate solutions. A TEM of an aliquot taken 24 h after mixing revealed spherical structures (approximately 300 nm in diameter), similar in appearance to those noted above. The only other entity present in this sample was an amorphous network of very small particles, 8–25 nm in diameter. After 48 h, plate-like crystals were present in addition to the spherical structures and small particles (Fig. 5), and after 72 h, neither the small particles nor the spherical structures could be observed in the reaction solution. DLS measurements (Table 2) on the initial reactant solutions gave mean particle diameters of: calcium 5.12 nm (s.d. 0.13) and phosphate 6.44 nm (s.d. 0.04). Substituting with distilled water resulted in a mean particle diameter of 6.8 nm (s.d. 0.08).
 | ||
| Fig. 5 Calcium phosphate structures present after 48 h of reaction where the w value was increased to 6.9. Elongated plate-like crystals of calcium hydrogen phosphate dihydrate, networks of amorphous nanoparticles, and ∼300 nm octacalcium phosphate spheres are observed. Scale bar = 400 nm. | ||
Hollow spheres with diameters in the range 40–125 nm (Fig. 6) were present after 72 h in solutions containing the highest w value (w = 16.2) investigated, and the greatest molar ratio of m-xylene (no isooctane). The spheres were apparent using TEM after just 24 h, and the wall thickness of the largest spheres was approximately 15 nm (measured from TEM micrographs). The background of the grids contained numerous tiny particles (5–10 nm in diameter), some of which also appeared to be hollow. Energy dispersive X-ray analysis confirmed that the spheres contained calcium and phosphate, and electron diffraction suggested that they were amorphous. TEM of an aliquot taken after 6 h showed only the spheres. DLS (Table 2) gave mean particle diameters of 6.04 nm (s.d. 0.19), 7.34 nm (s.d. 0.21), and 7.42 nm (0.25) for the initial calcium solution, phosphate solution, and water only respectively. However, a value of 68 nm (s.d. 19) was obtained for the reaction mixture 30 minutes after combining the two ion-containing solutions.
 | ||
| Fig. 6 Hollow spheres of amorphous calcium phosphate were synthesized from solutions with the greatest w value investigated, w = 16.2, and utilizing m-xylene as the only solvent. Scale bar = 200 nm. | ||
Control samples synthesised under each of the conditions described above, but lacking the AOT surfactant, all consisted of randomly orientated plate-like crystals of hydroxyapatite, similar to those displayed in Fig. 1c.
Discussion
Results of DLS and SAXS experiments (Table 2), along with a consideration of the ternary phase diagram for AOT/water/isooctane,38 suggest that initial calcium and phosphate solutions examined consist of reverse micelles or microemulsions. The mean diameter of these micelles/microemulsions, however, increases with the w value (Tables 1 and 2). This finding reflects a decrease in the strength of the interaction between the ions and surfactant as the molar ratio of water increases, and that of the surfactant decreases. The solutions that gave rise to the filamentous networks were not examined by DLS or SAXS, due to high viscosities rendering them unsuitable for DLS experiments, and our limited access to SAXS equipment. However, the AOT/water/isooctane phase diagram again predicts the presence of reverse micelles under these conditions.As described in previous studies, when two separate solutions containing AOT-stabilized reverse micelles/microemulsions are mixed, their aqueous contents exchange due to the dynamic nature of the system.37 Nucleation and growth of an inorganic material can then proceed within the mini-reaction vessels provided by the confinements of the organic structure. However, as is evident from the results presented here and elsewhere,37 the final product can vary greatly, and is dependent on subsequent events, dictated by parameters such as the ratio of inorganic cation to anion, the solvent used, molar ratios of water and surfactant, and the surfactant character.
Given the nature and molar ratios of the reagents used in this study, it is reasonable to expect a relatively high degree of interaction between the anionic surfactant head group and calcium ions, and subsequently with the growing inorganic species. Thus, crystal type, morphology, growth kinetics, and organization should be affected, resulting in final products that differ from controls (Fig. 1c), as we have observed.
In addition, previous studies have shown that the presence of salts in anionic surfactant solutions can have a dramatic effect on surfactant assembly and organization.40,41 For example, binding of cations such as Ca2+ to micellar/solution interfaces (Stern layer) can screen electrostatic repulsion between head groups of surfactants, thereby allowing micelle formation, growth, and rearrangement to form liquid crystalline phases. High surfactant concentrations (with respect to water) also favor the formation of lamellar liquid-crystalline phases. However, entropic gain through release of ions bound to micelles is another important factor in determining the dominant phase under a particular set of conditions.
The presence of small quantities of certain hydrophobic molecules (e.g. xylene) has also been shown to affect organization in surfactant solutions, by increasing the effective hydrophobic volume of the surfactant, thereby altering the critical packing parameter.42 This phenomenon may also perturb inorganic/organic interactions and affect the nature of inorganic materials synthesized in such solutions.
The reaction solution that results in enamel-like bundles of filaments contained a relatively high molar ratio of AOT with respect to water, calcium, and phosphate ions (w = 1.4), and was highly viscous. SAXS data (Table 2) show that before mixing the calcium and phosphate solutions, reverse micelles were likely to be present. However, the high viscosity of the reaction solution, in the absence of well-defined SAXS peaks, would be consistent with the presence of entangled rod-like micelles at these relative concentrations.38
It would appear from the TEM time study of the formation of the enamel-like bundles that 350–500 nm spherical structures and beam sensitive nanoscale particles form initially in the reaction solution, and are subsequently transformed into the bundles of filaments. Spherical structures similar in appearance to those seen here have been observed previously and identified as spherical octacalcium phosphate aggregates of thin plates. Such particles can nucleate rapidly on the surface of reaction containers or in areas of localized phase separation in solutions for example. These structures are not thermodynamically favored, however, and tend to transform into more stable crystal forms over time, as we have observed. The larger (500–800 nm) spherical particles of lower abundance are also octacalcium phosphate aggregates and transformed similarly. The amorphous nanoscale particles observed after 48 h are stabilized temporarily as a result of strong surfactant binding to their exterior, under conditions where very little water is present.
Thus, the nucleation and growth of bundles of hydroxyapatite crystals comprising the final product appears to take place initially within the constraints of entangled rod-like surfactant micelles. As the mineral continues to grow, rearrangement of the rod-like micelles is necessary to accommodate the increase in the inorganic length, and diameter in particular. Surfactant head groups may however continue to interact strongly with the crystal faces in the presence of excess calcium ions; directing mineral growth and leading to the formation of elongated hydroxyapatite crystals. Such a mechanism has been suggested previously for the formation of inorganic materials possessing high aspect ratios, as produced here, when synthesized in the presence of anionic surfactants such as AOT.34
The bundle formation observed in our experiments may be encouraged by the anisotropic nature/high concentration of rod-like surfactant micelles in the initial solution. The organization may proceed through interdigitation of the hydrophobic surfactant tails on adjacent hybrid rods, driven by the high concentrations of AOT in the presence of isooctane/m-xylene solvents, previously shown to promote the formation of higher-order phases such as a reverse hexagonal phase.43 The individual threads detected in the background during TEM observations could result either from the breakdown of bundles of filaments during sample preparation, or via a mechanism whereby AOT molecules adsorb onto selective surfaces of growing calcium phosphate particles, but fail to assemble into bundles.
Through these observations we have demonstrated that a salient feature of the enamel structure can be generated in vitro using an artificial system. Although the experimental conditions used here differ substantially from those present in the developing enamel matrix, it may be envisaged that a similar mechanism is involved in the formation of enamel in vivo. Amelogenin may play a similar role to that of AOT in the control of amorphous calcium phosphate formation in a compartmentalized fashion, and in the subsequent regulation of co-aligned crystal bundle formation seen during early enamel formation. Consistent with this suggestion, based on an analysis of both in vivo and in vitro findings, it has been proposed that amelogenin can form spherical subunits (nanospheres) that stabilize mineral precursors (possibly amorphous calcium phosphate) which, upon proteolytic processing, subsequently fuse to form long hydroxyapatite crystals.10 This latter mechanism, however, does not explain the formation of parallel arrays of crystals as seen here and in developing enamel. However, the present results are also in line with other recent in vitro findings (reviewed in ref. 22) that have elegantly demonstrated in a variety of systems that interplay between the organization of organic molecules and crystallization, and the subsequent cooperative reorganization of hybrid inorganic–organic nanostructures, can give rise to the formation of hierarchical structures. These latter findings lead these authors to suggest that similar cooperative processes may be involved in the formation of biominerals. In further support of this hypothesis, and consistent with the results obtained in the present study, we have recently found through in vitro studies using recombinant amelogenins that bundles of co-aligned calcium phosphate crystals can be formed, but only when crystal formation and amelogenin assembly occur simultaneously.44 This observation suggests that these latter events must occur in a cooperative fashion to obtain a highly ordered product. The present data provide additional support for this hypothesis, based on direct evidence of changes in both organic template and inorganic structures as a function of time.
The nature of the products formed was highly sensitive to variations in the molar ratios of the components in the reaction mixtures. Interconnecting filamentous networks of hydroxyapatite (Fig. 3) were formed by increasing the molar ratio of m-xylene and slightly reducing the AOT molar ratio, with respect to the composition used for the formation of the bundles of filaments (Table 1). Once again, if the initial solutions contained reverse micelles/microemulsions, rearrangement of the organic phase at some stage would be necessary in order to obtain a product with the dimensions exhibited. These networks may also arise from nucleation and early growth within entangled rod-like micelles. In this case, however, the rods remain entangled as the crystals develop, leading to a continuous network structure of co-aligned crystals, rather than isolated bundles as seen in Fig. 1a. The persistence of micelle entanglement may be due to the presence of longer rod-like micelles in this reaction medium. This could be a result of the increased xylene concentration present, shifting the balance in favor of larger surfactant aggregates/structures. The formation of such a product would still be highly dependent on selective adsorption of the surfactant to specific crystal faces of the inorganic species as the reaction proceeds. This type of structure may hold potential for use as a biological implant material, for example.
The conditions that give rise to amorphous nanoparticles of calcium phosphate involve a decrease in the molar ratio of AOT in the reaction mixture (w = 2.4), and a substantial decrease in viscosity. The presence of reverse micelles and a microemulsion are still evident in the initial reaction solutions according to DLS data, and the low viscosity of these solutions suggests that entangled rod-like micelles are not present. In this case, the product most likely develops within spherical surfactant micelles. Reverse micelles and microemulsions have been used previously for the synthesis of discrete inorganic nanoparticles of controlled size distribution.37 The surfactant caps the surface of primary inorganic particles, preventing their further growth. Interdigitation of the alkyl chains of the surfactant leads to bilayer formation, and enhanced stability of the nanoparticles. Methods for preparing inorganic nanoparticles are of interest as such particles possess intriguing size dependent properties, giving them the potential for use in a wide range of applications.
The formation of calcium hydrogen phosphate dihydrate crystals under conditions where the molar ratio of AOT is reduced substantially (w = 6.9) again illustrates the notion that preferential surfactant adsorption can inhibit the growth of a thermodynamically favored crystal type (i.e., hydroxyapatite), thereby promoting the nucleation of an alternative kinetically favored phase. Under the conditions of this experiment, calcium hydrogen phosphate dihydrate is the preferential phase. This product may again form through rapid conversion of amorphous calcium phosphate, which could not be stabilized by the lower molar ratio of AOT present.45 Elongation of the calcium hydrogen phosphate dihydrate crystal plates further suggests preferential adsorption of AOT on specific crystal faces. This observation has been noted previously.33,34 TEM images of aliquots taken during the reaction again suggest the presence of octacalcium phosphate in the solution, which subsequently transforms into calcium hydrogen phosphate dihydrate.
Hollow spheres were produced under conditions where the molar ratio of AOT was the lowest (w = 16.2). This synthesis also employed m-xylene as the only hydrophobic solvent. A possible mechanism for the formation of the spheres may involve deposition of amorphous calcium phosphate onto the surface of water droplets, with no interference by the surfactant under these conditions. DLS data [not shown] suggest the presence of relatively large structures in the reactant solution 30 minutes after mixing, but smaller reverse micelles/microemulsions in the initial calcium and phosphate solutions. The additional water present in this solution with respect to AOT, along with the change in solvent, may allow the development of relatively large water droplets in the medium. Hollow spheres of calcium phosphate hold potential as drug delivery vehicles, for example, and have biocompatibility advantages over other materials.
Of particular note in our experiments is the fact that a highly viscous reaction medium was used to produce the co-aligned bundles of hydroxyapatite crystals. Such structures are absent when solutions of low viscosity were employed, where discrete amorphous calcium phosphate particles were formed. Although other factors clearly come into play, a partial explanation for these findings is that high viscosity can prevent long range diffusion and result in local conditions that favor the formation of anisotropic structures, as observed here. In contrast, under conditions of low viscosity and unrestricted diffusion, uniform nucleation and growth of amorphous calcium phosphate was observed. Thus, the viscous nature of the amelogenin gel matrix in developing enamel may serve to favor the formation of anisotropic structures, as seen in the generation of parallel arrays of enamel crystals during the initial stages of dental enamel mineralization.
In conclusion, the surfactant AOT has been used to control the growth of calcium phosphates. A variety of materials have been formed, possessing a number of morphologies and crystal types. By varying the molar ratio of water to surfactant, consequent inorganic–surfactant head group interactions, and with the use of small amounts of m-xylene co-solvent, the final product has been tailored in a number of ways. It is evident that the surfactant has a strong influence on the growth of the inorganic material. Of particular interest, bundles of co-aligned hydroxyapatite fibers have been synthesized. These bundles are formed from highly viscous solutions and possess structural similarities to dental enamel. In living systems, proteins and other biomolecules provide more elaborate template/structure directing agents, which result in more complex mineral structures. However, simplified invitro systems such as those described here can provide useful information regarding the mechanisms involved in the formation of biominerals invivo. Importantly, we have provided new evidence in support of the hypothesis that hierarchical structures found in nature may result from cooperative interactions between organic assembly and crystal growth. In addition, our findings suggest that biological systems may similarly utilize viscous reaction environments to promote the formation of highly anisotropic predominantly inorganic structures.
Acknowledgements
We would like to thank Barbara Aichmayer of the Institute of Metal Physics, University of Leoben, Austria, and Dr Peter Fratzl of the Max Planck Institute of Colloids and Interfaces, for their help with SAXS experiments, and Dr Elia Beniash for his valuable comments on this manuscript. We also acknowledge the support of this work by grant PO1-DE-13237 (HM) from the National Institute of Dental and Craniofacial Research (NIDCR).References
- J. K. Avery, R. L. Visser and D. E. Knapp, J. Dent. Res., 1961, 40, 1004.
- A. G. Fincham, J. Moradian-Oldak and J. P. Simmer, J. Struct. Biol., 1999, 126, 270–299 CrossRef CAS.
- J. D. Termine, A. B. Belcourt, P. J. Christner, K. M. Conn and M. U. Nylen, J. Biol. Chem., 1980, 255, 9760–9768 CAS.
- C. W. Gibson, Z. A. Yuan, B. Hall, G. Longenecker, E. Chen, T. Thyagarajan, T. Sreenath, J. T. Wright, S. Decker, R. Piddington, G. Harrison and A. B. Kulkarni, J. Biol. Chem., 2001, 276, 31871–31875 CrossRef CAS.
- A. G. Fincham, J. Moradian-Oldak, T. G. Diekwisch, D. M. Lyaruu, J. T. Wright, P. Bringas, Jr. and H. C. Slavkin, J. Struct. Biol., 1995, 115, 50–59 CrossRef CAS.
- J. Moradian-Oldak, J. P. Simmer, E. C. Lau, P. E. Sarte, H. C. Slavkin and A. G. Fincham, Biopolymers, 1994, 34, 1339–1347.
- M. Iijima, Y. Moriwaki, T. Takagi and J. Moradian-Oldak, J. Cryst. Growth, 2001, 222, 615–626 CrossRef CAS.
- H. B. Wen, J. Moradian-Oldak, W. Leung, P. Bringas, Jr. and A. G. Fincham, J. Struct. Biol., 1999, 126, 42–51 CrossRef CAS.
- J. Moradian-Oldak, Matrix Biol., 2001, 20, 293–305 CrossRef CAS.
- C. Robinson, R. C. Shore, S. R. Wood, S. J. Brookes, D. A. M. Smith, J. T. Wright, S. Connell and J. Kirkham, Connect. Tissue Res., 2003, 44, 65–71 CAS.
- D. Walsh, J. D. Hopwood and S. Mann, Science, 1994, 264, 1576–1578 CrossRef CAS.
- H. T. Schmidt and A. E. Ostafin, Adv. Mater., 2002, 14, 532–535 CrossRef CAS.
- M. Antonietti, M. Breulmann, C. G. Goltner, H. Colfen, K. K. W. Wong, D. Walsh and S. Mann, Chem. Eur. J., 1998, 4, 2493–2500 CrossRef CAS.
- H. Colfen and L. M. Qi, Chem. Eur. J., 2001, 7, 106–116 CrossRef CAS.
- J. P. Roman, P. Hoornaert, D. Faure, C. Biver, F. Jacquet and J. M. Martin, J. Colloid Interface Sci., 1991, 144, 324–339 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- F. J. Arriagada and K. Osseoasare, Colloids Surf., 1992, 69, 105–115 CrossRef CAS.
- M. Breulmann, H. Colfen, H. P. Hentze, M. Antonietti, D. Walsh and S. Mann, Adv. Mater., 1998, 10, 237–+ CrossRef CAS.
- F. C. Meldrum, B. R. Heywood and S. Mann, Science, 1992, 257, 522–523 CrossRef CAS.
- S. H. Yu and H. Colfen, J. Mater. Chem., 2004, 14, 2124–2147 RSC.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684–1688 CrossRef CAS.
- H. Colfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef.
- R. Gonzalez-McQuire, J. Y. Chane-Ching, E. Vignaud, A. Lebugle and S. Mann, J. Mater. Chem., 2004, 14, 2277–2281 RSC.
- R. Norenberg, H. Sterzel and V. Koch, Rod Shaped Apatite Crystals with a Specific Length-to-Width Ratio, US Pat. 20040171471A1, 9/2/2004.
- D. Walsh, J. L. Kingston, B. R. Heywood and S. Mann, J. Cryst. Growth, 1993, 133, 1–12 CrossRef CAS.
- D. Skrtic and N. Filipovic-Vincekovic, J. Cryst. Growth, 1988, 88, 313–320 CrossRef CAS.
- L. Tunik, L. Addadi, N. Garti and H. Füredi-Milhofer, J. Cryst. Growth, 1996, 167, 748–755 CrossRef CAS.
- M. J. J. M. Vankemenade and P. L. Debruyn, Colloids Surf., 1989, 36, 359–368 CrossRef CAS.
- M. Li and S. Mann, Adv. Funct. Mater., 2002, 12, 773–779 CrossRef CAS.
- M. Li, B. Lebeau and S. Mann, Adv. Mater., 2003, 15, 2032–2035 CrossRef CAS.
- T. Aoba, M. Fukae, T. Tanabe, M. Shimizu and E. C. Moreno, Calcif. Tiss. Int., 1987, 41, 281–289 Search PubMed.
- T. Aoba and E. C. Moreno, in Surface reactive peptides and polymers, ed. C. S. Sikes and A. P. Wheeler, American Chemcial Society, Washington DC, 1991, pp. 85–106 Search PubMed.
- M. Bujan, M. Sikiric, N. Filipovic-Vincekovic, N. Vdovic, N. Garti and H. Furedi-Milhofer, Langmuir, 2001, 17, 6461–6470 CrossRef CAS.
- S. Sarda, M. Heughebaert and A. Lebugle, Chem. Mater., 1999, 11, 2722–2727 CrossRef CAS.
- A. López-Macipe, J. Gómez-Morales and R. Rodríguez-Clemente, J. Cryst. Growth, 1996, 166, 1015–1019 CrossRef CAS.
- G. D. Rees, R. Evans-Gowing, S. J. Hammond and B. H. Robinson, Langmuir, 1999, 15, 1993–2002 CrossRef CAS.
- M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402, 393–395 CrossRef CAS.
- H. Kunieda and K. Shinoda, J. Colloid Interface Sci., 1979, 70, 577–583 CrossRef CAS.
- M. J. Glimcher, E. J. Daniel, D. F. Travis and J. Kahmi, J. Ultrastruct. Res., 1965, 50, SUPPL-77 Search PubMed.
- A. Sein, J. B. F. N. Engberts, E. Vanderlinden and J. C. Vandepas, Langmuir, 1993, 9, 1714–1720 CrossRef CAS.
- A. Sein and J. B. F. N. Engberts, Langmuir, 1995, 11, 455–465 CrossRef CAS.
- H. Hoffmann and W. Ulbricht, J. Colloid Interface Sci., 1989, 129, 388–405 CrossRef CAS.
- K. Aramaki, A. Akahane and H. Kunieda, Progress in Colloid and Polymer Science, Springer-Verlag, Heidelberg, 2003, pp. 40–43 Search PubMed.
- E. Beniash, J. P. Simmer and H. C. Margolis, J. Struct. Biol., 2005, 149, 182–190 CrossRef CAS.
- R. E. Wuthier, G. S. Rice, Jr. J. E. B. Wallace, L. Weaver, R. L. LeGeros and E. D. Eanes, Calcif. Tiss. Int., 1985, 37, 401–410 Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |
