Bioadhesion at micro-patterned stimuli-responsive polymer brushes†
Carolina de las Heras Alarcóna, Tamer Farhanb, Vicky L. Osborneb, Wilhelm T. S. Huckb and Cameron Alexander*c
aSchool of Pharmacy and Biomedical Sciences, Institute of Biomedical and Biomolecular Sciences, University of Portsmouth, Portsmouth, UK PO1 2DT. Fax: +44 (0)23 9284 3565; Tel: +44 (0)23 9284 3598
bMelville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK CB2 1EW
cSchool of Pharmacy, Boots Science Building, University of Nottingham, University Park, Nottingham, UK NG7 2RD. E-mail: cameron.alexander@nottingham.ac.uk; Fax: +44(0)115 951 5102; Tel: +44(0)115 951 5100
First published on 28th April 2005
Abstract
The synthesis of poly(N-isopropylacrylamide) brushes within micropatterned domains at surfaces and the performance of these functionalised surfaces in short-term bioadhesion assays under varying conditions are described. The polymer brushes show temperature dependent behaviour at surfaces as demonstrated by changes in contact angle, surface energy components and aqueous phase AFM. The responses in the polymer brush domains result in spatially defined, and temperature mediated, attachment of a model protein, BSA, and the common oral bacteria Streptococcus mutans.
Introduction
The preparation of polymer brush layers within spatially-defined domains by microcontact printing and surface initiated polymerisation offers a versatile route to robust ‘smart’ surfaces.1 Patterned stimuli-responsive polymer brushes are of particular appeal because a single surface can contain multiple chemical and functional environments that can be switched in specified regions by an external stimulus. Responsive brushes of poly(N-isopropylacrylamide) (PNIPAm), which display coil-to-globule transitions at a lower critical solution temperature (LCST, 32 °C for PNIPAm), are being investigated owing to their wide spectrum of useful properties.2–5 Patterned PNIPAm materials are especially useful for investigating the interactions of biological species at surfaces, since the effects of the polymer response on biopolymer or cell attachment can be evaluated at the brush domains in comparison with the non-responsive regions, which act as internal controls. In this study we demonstrate that a model protein and a specific bacterial strain exhibit variable attachment to micropatterned PNIPAm domains at a surface dependent on polymer brush response.Materials and methods
Chemicals and biological media
N,N,N′,N′,N″-Pentamethyldiethylenetriamine, (PMDETA); 2,2′-dipyridyl (bpy); hexadecanethiol (HDT), copper(I) bromide, copper(II) bromide and N-isopropylacrylamide were all supplied from Aldrich. Standard reagents and chemicals were purchased from Fisher Scientific or Aldrich and used as received. Bovine serum albumin (BSA) (Mw 66 kDa, pI 5.2), fluorescein isothiocyanate (FITC), ethidium bromide and buffers 3-N-morpholinopropanesulfonic acid, (MOPS), EDTA, and sodium acetate buffer were purchased from Sigma. Streptococcus mutans 10499 cultures were supplied by Mr David Churchley, University of Portsmouth. Horse blood agar, nutrient agar sterile brain heart infusion and sterile single-strength nutrient broth were purchased from Oxoid.Polymer brush synthesis
Microcontact printed initiator and HDT patterns were prepared as described previously.5 Polymer brush growth was achieved by placing the gold substrates in Schlenk tubes under a N2 atmosphere and adding a degassed solution of NIPAM (25.2 g, 0.22 mol), Cu(I)Br (0.32 g, 2.22 mmol), and PMDETA (1.4 mL, 6.66 mmol) in H2O (12.6 mL) and MeOH (2.6 mL) via a cannula tube: these amounts of monomer and catalysts represented a large excess relative to surface bound initiator. The polymerisation was allowed to proceed for a set reaction time (1–150 min) at room temperature, after which the substrates were removed from the reaction flask and the reaction was stopped. The polymerisation mixture was poured into ice-cold MeOH, but no precipitate or other evidence for polymerisation in solution was observed. The substrates were rinsed extensively with H2O and MeOH to remove all traces of the polymerisation bath.Bioadhesion studies
All solutions for biological assays were sterilized prior to use.Protein adsorption
Fluorescein isothiocyanate (FITC)-labelled BSA (0.2 mg mL−1, typically 37.5 µL) was added to MOPS (50 mM, pH 7.0, 10 mL) in 15 mL screw capped bottles. Patterned PNIPAm surfaces were transferred to the protein solutions. The bottles were incubated, with gentle shaking, for 1 h at the assay temperature, rinsed twice in MOPS (50 mM, pH 7.0, 10 mL) and then with purified water at the same temperature. Longer incubation time assays employed the same rinse and imaging procedures. Imaging of the samples was carried out via conventional and confocal laser scanning microscopies.Bacterial adsorption
Stock cultures of Streptococcus mutans 10499 were maintained on horse blood agar and nutrient agar (Oxoid) respectively. A vial containing sterile brain heart infusion (27 mL) was inoculated directly from the colonies and incubated at 37 °C for 24 h. The bacterial cultures were stained with ethidium bromide (10 mg per 27 mL of broth) and further incubated for 3 h at 37 °C. After this time the broths were transferred to centrifuge tubes and centrifuged at 3000 rpm for 5 min. The bacteria were washed twice in sterile MOPS (50 mM, pH 7.0, 10 mL), and finally re-suspended in MOPS (5 mL). To four 20 mL universal bottles containing sterile MOPS buffer (4 mL) was added the re-suspended culture (1 mL). Polymer brush surfaces were added to these universal bottles, which were incubated above and below polymer LCST and through multiple temperature and time cycles.The surfaces were rinsed twice by immersion in sterile MOPS (10 mL), at the assay temperature and then were observed by fluorescence microscopy and AFM. All experiments were conducted in triplicate.
Contact angle goniometry
A Kruss G10 contact angle measuring system equipped with a sealed sample chamber, and automated image analysis system was used. Drops of liquid of known volume (1–4 µL) were applied from a micro-syringe to the surface of the test material through a small port at the top of the cell: to avoid cross-contamination of liquids, a dedicated micro-syringe was used for each diagnostic liquid. The precision of the angle measurement was ±0.5°. Advancing and receding water contact angles were measured for polymer brush surfaces at temperatures below LCST (typically 12–20 °C and at 40 °C above LCST). Surface energies were calculated from advancing contact angles values obtained at both temperatures using double deionised water (DDW), diiodomethane (DIM) and ethylene glycol (EG) using the method of van Oss et al.6 Polymer brush surfaces were exposed to DDW, DIM and EG under vapour-saturated atmospheres of individual liquids, and advancing and receding contact angles were measured from ten different drops on the same sample.Ellipsometry and infra-red spectroscopy
Ellipsometric measurements were carried out on a DRE ELX-02C ellipsometer with a 632.8 nm laser at 70° from the normal.FT-IR spectra were recorded on a BioRad FTS 6000 spectrometer.
Atomic force microscopy
Atomic force microscopy (AFM) studies were carried out using a TopoMetrix TMX2000 Discoverer Scanning Probe Microscope (ThermoMicroscopes, UK) with a 70 × 70 × 12 µm tripod piezoelectric scanner, or a Digital Instruments MultiMode equipped with a NanoScope IV controller (Veeco Instruments, Santa Barbara, CA, USA). Topography measurements were conducted using V-shaped silicon nitride cantilevers bearing an integrated standard profile tip (length 200 µm, nominal spring constant (K) 0.032 N.m−1; ThermoMicroscopes, Santa Clara, CA, USA). Topographic imaging was performed in air and in water using a closed wet cell, modified to allow variable temperature adjustment. Contact mode imaging utilised an applied load and scan rate limited to ca. 1 nN and 3 Hz, respectively, to minimize compression and lateral damage to polymers and underlying surfaces. For the Nanoscope IV instrument, imaging was performed both in air and in water using tapping mode and an E-scanner (xyz = 13 × 13 × 10 µm). In air, beam-shaped silicon cantilevers with a nominal spring constant and resonant frequency of 31–71 N m−1 and 170–210 kHz, respectively (PointProbes, type NCL-16, NanoSensors, Germany) were used. For studies performed in water (0.2 µm filtered, 18 MΩ; Sigma, Dorset, UK), oxide-sharpened, silicon nitride, V-shaped cantilevers with a spring constant and resonant frequency of 0.12 N m−1 and 7–9 kHz (NanoProbe tips, Veeco Instruments, Santa Barbara, CA, USA) were used. Images were digitally levelled, although no further processing was used. AFM topography imaging under water of patterned PNIPAm brushes was performed at temperatures above and below the solution LCST of PNIPAm (32 °C). Polymer brush thicknesses were measured in contact mode AFM experiments. The PNIPAm brushes were scanned in water starting at 10 °C and the temperature was raised gradually to 40 °C. Images were obtained at the two temperatures of the same area, which was rescanned, and brush heights from each image were calculated. As a control the topographic scans were repeated at a constant temperature over the original area, and only a small change in brush height was observed (∼4 nm). The same AFM tip was used in all these measurements. Adhesion force mapping was carried out above and below LCST with force distance curves obtained from pull off forces of the AFM tip in water over the PNIPAm brushes and HDT domains.Confocal laser scanning microscopy
A Zeiss LSM 510 Meta instrument, fitted with argon and He–Ne lasers operating at excitation wavelengths of 453, 488 and 543 nm, using emission filters of 505–530 and 585–615 nm, was used for evaluation of dye-labelled protein and cell attachment at surfaces.Cell attachment: quantification and image analysis
Analysis of confocal micrographs was conducted for three fields chosen at random on each surface using image analysis software (Scion Image). Line profiles (nominal 100 µm length) were drawn using the software's Profiling Tool and greyscale intensities recorded along PNIPAm brushes, along HDT domains, and across brush and SAM areas: only cells lying wholly within the PNIPAm domains as evidenced by microscopy were assigned as attached to PNIPAm. Image intensities were converted to bacterial counts using the analyse particles tool, based on image intensities of individual cells at selected points on each micrograph.Results and discussion
The brushes were grown via surface-initiated atom transfer radical polymerization (ATRP) from micro-contact printed self-assembled monolayers as shown in Fig. 1. | ||
| Fig. 1 Microcontact printing of gold surfaces and surface-initiated ATRP of N-isopropylacrylamide. | ||
Poly(N-isopropylacrylamide) (PNIPAm) brushes of over 250 nm height (ellipsometry) in dry air could be prepared via this route within 2 h reaction time. Grazing angle FT-IR spectra confirmed the functionality of the polymer brushes, with characteristic absorptions at 3320 cm−1 (N–H stretch), 2974 cm−1 (C–H stretch), 1650, 1533 cm−1 (amide I and II) and the isopropyl methyl deformation bands at 1367 and 1386 cm−1. AFM indicated that addition of water below polymer LCST caused swelling of the brushes while hot water caused a contraction of the brush thickness by ∼80 nm. Control of film thickness was obtained by diluting the initiator within the brush domains with an inert thiol, or by carrying out brush synthesis for shorter times (Table 1) as the relationship between the polymerization time and brush thickness could be reasonably predicted. The data further implied that brushes above 50% initiator composition apparently reached a maximum density, where the increase in initiator moieties on the surface no longer resulted in an increase in thickness of the polymer film.
| Initiator density (%) | Film thickness/nm | Polymerization time/min | Film thickness/nm |
|---|---|---|---|
| 10 | 57 | 1 | 89 |
| 25 | 99 | 6 | 107 |
| 50 | 254 | 10 | 114 |
| 100 | 266 | 32 | 221 |
The relation between film thickness and polymerisation time was non-linear, with termination occurring within 30 min of the polymerisation indicating that brush growth was not living. This correlated with previous work on ATRP of other acrylamides,7 which showed that polymerisation was not fully controllable most likely due to stabilisation of growing radical chain ends via coordination of copper to the amide groups. This data, and the absence of additional IR bands other than those of PNIPAm, also suggested hydrogen chain-ends of the brushes as a consequence of termination and stabilisation of the growing radical chains.
For the bulk of our assays we concentrated on PNIPAm brushes of ∼55 nm height as measured by aqueous phase AFM8 in domains of 5 µm width with the remainder of the surface composed of hexadecanethiol (HDT) (Fig. 2).
 | ||
| Fig. 2 AFM topography of PNIPAm brushes (a) polymer brushes below LCST with (b) z profile. | ||
Equilibrium water contact angle variations9 provided evidence for the coil–globule transitions of PNIPAm around the lower critical solution temperature (LCST, 32 °C for PNIPAm), while AFM adhesion forces measured in aqueous solutions (0.5–1.5 nN below LCST, 3.3–3.7 nN above LCST) confirmed the hydrophilic–hydrophobic switch. The reversibility of polymer response was shown in further aqueous phase AFM topography images, with a change in average brush layer thickness over the LCST transition (Table 2).
| T/°C | θw/° Water | γs(LW) | γs+ | γs− | γstot/mJ m−2 | Z/nm |
|---|---|---|---|---|---|---|
| 12 | 65.5 ± 12.8 | 38.17 | 1.14 | 30.78 | 50.03 | 50 ± 5 |
| 40 | 89.8 ± 3.7 | 34.59 | 0.13 | 2.04 | 35.63 | 27 ± 2 |
Surface energy components6 showed a marked reduction in the Lewis base contribution γs− over the polymer phase transition. Previous results have suggested that surfaces with high Lewis base components should exhibit low protein adsorption,10,11 which, if the proteins are able to form conditioning layers, is considered to be the first stage in bacterial adhesion and fouling. We therefore challenged the patterned surfaces with solutions of a model protein, FITC-labelled BSA, to assess the brush response prior to bacterial adhesion assays. Confocal microscopy (Fig. 3) showed reduced BSA adsorption to the PNIPAm domains when the polymer brush surfaces were incubated with FITC-BSA for 1 h above LCST but rinsed below their LCST compared to surfaces when the rinse cycle was carried out above LCST.12 Area corrected estimates based on evaporated deposits from known solutions indicated that 0.7 ± 0.2 pmol cm−2 FITC-BSA remained attached after a rinse below LCST in these experiments, compared to 1.4 ± 0.4 pmol cm−2 when rinsed above LCST (n = 6, z test two sample for means, P = 0.12).12 These results were in accord with previous reports of increased protein adsorption to PNIPAm surfaces above LCST.13–15 Incubation assays in which the temperatures were cycled below and above LCST over longer timescales (6–72 h) showed a rather more complex pattern of adsorption, with a change in protein localisation at the surfaces over time and partial accumulation at brush–HDT boundaries. This suggested that longer term adsorption was not only dependent on surface physico-chemistry, but also affected by factors such as protein–polymer H-bonding interactions, steric exclusion,16 surface disorder17 and protein denaturation.18
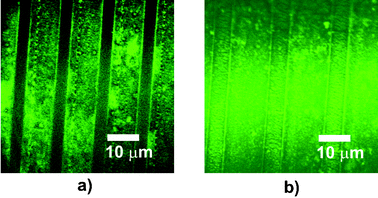 | ||
| Fig. 3 Confocal microscopy of FITC-BSA attachment to surfaces after incubation with FITC-BSA at 37 °C and rinse at 12 °C (a) or rinse at 40 °C, (b). Dark areas in (a) indicate hydrophilic PNIPAm brushes with little or no attached FITC-BSA whereas in (b), overall fluorescence shows adsorbed FITC-BSA at both hydrophobic PNIPAm tracks above LCST and HDT. | ||
The attachment of bacteria at surfaces is less rapid than that of proteins, and we therefore reasoned that the surface response over 1 h incubation periods might also affect adsorption of microorganisms. In order to test this hypothesis we carried out attachment assays with Streptococcus mutans. This strain was selected as it is a common oral pathogen, of coccoid morphology, and also because it has been reported to demonstrate higher adhesion to hydrophilic rather than hydrophobic substrates.19 This is in contrast to many other biological moieties; bacteria such as Salmonella typhmurium and Bacillus cereus attach more readily to hydrophobic surfaces (including PNIPAm) above LCST.10 Specifically we were interested in whether PNIPAm brush domains might reversibly adsorb S. mutans over cycles of polymer coil–globule collapse. This would demonstrate that the polymer phase transition response was the most important factor controlling initial cell attachment rather than the inherent chemical functionality of the brush.
PNIPAm/HDT surfaces (∼1 cm2) were incubated with suspensions (108 cfu mL−1) of ethidium bromide stained S. mutans at 37 °C and 4 °C over repeat cycles and the surfaces were rinsed twice prior to fluorescence microscopy. Initial incubation of S. mutans at 37 °C for one hour resulted in non-selective adsorption across the surface (Fig. 4a). The same samples were incubated with another culture at 4 °C (Fig. 4b) leading to cell accumulation at the now hydrophilic PNIPAm brushes indicating the higher cell surface affinity at these domains. After a third hour the samples were again transferred into another culture and incubated at 37 °C (Fig. 4c) causing loss of alignment of the bacteria and a more random pattern of attachment. The final stage was another hour at 4 °C, which resulted in concentration of the cells at the PNIPAm brushes again (Fig. 4d).
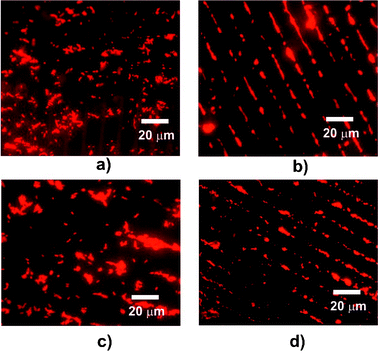 | ||
| Fig. 4 Fluorescence microscopy of micro-patterned surfaces incubated in suspensions of S. mutans. Images (three random fields) were recorded over two reversibility cycles. Images (a–d): initial incubation at 37 °C for 1 h (a) followed by incubation at 4 °C for 1 h (b), transfer to culture at 37 °C for 1 h (c) and a second incubation at 4 °C for 1 h (d). | ||
The analogous temperature cycling assay was also carried out but commencing at 4 °C for 1 h and, in this experiment too, selective adsorption to the PNIPAm areas took place when the brushes were below LCST. Evidence of increased overall bacterial retention on the surfaces was apparent after the second attachment cycle, suggesting that after more prolonged periods, cell-to-cell attachment and cross-surface bridging interactions were taking place. Nevertheless, the more hydrophilic PNIPAm domains retained the most bacteria, indicating the importance of relative affinity in short-term biosorption events.20
Bacterial counts averaged over all the cycles varied from 0.6 × 106 cfu cm−2 on PNIPAm above LCST to 1.2 × 106 cfu cm−2 for the same surfaces below LCST, whereas almost no difference in adsorption to the HDT domains was observed (<1.3 × 105 cfu cm−2) (Fig. 5a). This demonstrated that even after four assays and irrespective of the start temperatures the brush response retained an effect on bacterial attachment. Cell alignment on the PNIPAm brushes was also apparent in AFM images at the end of the assay cycles at 4 °C (Fig. 5b). AFM and confocal micrographs showed that the bacteria appeared to be attached in greater numbers in the centre of the brushes than at the edges: close inspection revealed that the bacteria were generally aggregated in these regions, particularly in the later stages of the experiments. This suggested that the apparent concentration of the cells was more likely a function of the greater difficulty in removing larger ‘clumps’ of cells in the rinse cycles than any preference for particular regions of the brush domain. In addition, the changes in fluid flow over the micropatterned surface layers may have resulted in more rapid removal of bacteria from brush edges during rinsing. Experiments to test this hypothesis with varying widths of PNIPAm tracks and different geometries are currently in progress.
 | ||
| Fig. 5 S. mutans attachment after two repeat incubation assays; (a) cell counts (microscopy) averaged over all surfaces, (b) atomic force micrograph after final incubation at 4 °C. | ||
Conclusions
Micropatterned poly(N-isopropylacrylamide) brushes were shown to exhibit the expected coil–globule phase transitions at surfaces, and these transitions were accompanied by a reduction in brush layer height above LCST. The PNIPAm domains decreased in overall surface energy above the transition and also displayed reduced Lewis basicity at the higher temperatures. The adsorption of a model protein to the PNIPAm regions was also LCST dependent over short timescales, although over prolonged time periods and with temperature cycling there was evidence for aggregated protein in the polymer tracks. Adsorption of Streptococcus mutans also varied with temperature, with differences in the number of cells attached to the PNIPAm domains. The change in the pattern of S mutans attachment following ‘cycling’ of PNIPAm brushes above and below the LCST was correlated with changes in the surface properties as a result of the phase transitions, since all other controllable factors (buffer strength, rinse cycles, HDT domain structure) were constant for all areas of the surfaces.21 No cell-specific affinity ligands were required to direct this preferred attachment to defined areas of the surface under lower temperature conditions.Overall the results suggest that fabrication of micro patterned materials with the appropriate features and responses to temperature and other stimuli may allow externally mediated control of biopolymer binding and release, or prokaryotic and eukaryotic cell attachment. This approach should lead to surfaces which can be further tuned to adsorb or reject particular cell types in a variety of arrays or patterns, and thus to more effective biocompatible materials, sensors and biomedical devices.
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC), the University of Portsmouth, ICI and the Isaac Newton Trust for financial support. We also thank Drs James Smith, Beverley Twaites and David Churchley (University of Portsmouth), for assistance with AFM and microbiology, and two referees for helpful and constructive comments.References
- S. Edmonson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14 RSC.
- Q. Fu, G. V. R. Rao, S. B. Basame, D. J. Keller, K. Artyushkova, J. E. Fulghum and G. P. Lopez, J. Am. Chem. Soc., 2004, 126, 8904 CrossRef CAS.
- S. Mendez, L. K. Ista and G. P. Lopez, Langmuir, 2003, 19, 8115 CrossRef CAS.
- S. Kidoaki, S. Ohya, Y. Nakayama and T. Matsuda, Langmuir, 2001, 17, 2402 CrossRef CAS.
- D. M. Jones, J. R. Smith, W. T. S. Huck and C. Alexander, Adv. Mater., 2002, 14, 1130 CrossRef CAS.
- C. J. van Oss, M. K. Chaudhury and R. J. Good, J. Am. Chem. Soc., 1988, 88, 927 CAS.
- J. T. Rademacher, M. Baum, M. E. Pallack and W. J. Brittain, Macromolecules, 2000, 33, 284 CrossRef CAS.
- Contact mode imaging AFM has been shown to report lower brush heights than ellipsometry owing to compression of the brush layer under the applied load of the AFM tip: see, for example, ref. 5.
- Changes in contact angle of overall higher magnitude (θw(12 °C) = 19°, θw(40 °C) = 87°) were evident for non-patterned PNIPAm surfaces suggesting that conventional drop analysis of patterned surfaces sampled both polymer brushes and the HDT background.
- R. G. Chapman, E. Ostuni, M. N. Liang, G. Meluleni, E. Kim, L. Yan, G. Pier, H. S. Warren and G. M. Whitesides, Langmuir, 2001, 17, 1225 CrossRef CAS.
- D. Cunliffe, C. de las Heras Alarcon, V. Peters, J. R. Smith and C. Alexander, Langmuir, 2003, 19, 2888 CrossRef CAS.
- HDT domains did not vary significantly in FITC-BSA adsorption with temperature over the 1 h experiments (1.6 ± 0.5 pmol cm−2 at 12 °C and 1.0 ± 0.2 pmol cm−2 at 37 °C). Contrast observable in confocal microscopy was most likely due to multilayer adsorption at and in the hydrophobic PNIPAm domains. Quantification of BSA was limited by self-quenching of protein multi-layers through the confocal sampling depth hence the relatively large experimental error: similar factors although of lesser magnitude also applied to cell adhesion assays.
- E. C. Cho, Y. D. Kim and K. Cho, Polymer, 2004, 45, 3195 CrossRef CAS.
- S. X. Cheng, J. T. Zhang and R. X. Zhuo, J. Biomed. Mater. Res., 2003, 67A, 96 CrossRef CAS.
- D. L. Huber, R. P. Manginell, M. A. Samara, B. I. Kim and B. C. Bunker, Science, 2003, 301, 352 CrossRef CAS.
- F. Fang and I. Szleifer, Biophys. J, 2001, 80, 2568 CrossRef CAS.
- J. Y. Fang and C. M. Knobler, Langmuir, 1996, 12, 1368 CrossRef CAS.
- W. Norde, Adv.Colloid Interface.Sci., 1986, 25, 267 CrossRef CAS.
- A. W. J. Van Pelt, H. C. Van der Mei, H. J. Busscher, J. Arends and A. H. Weerkamp, FEMS Microbiol. Lett., 1984, 25, 279 CrossRef.
- R. Bos, J. H. De Jonge, B. Van De Belt-Gritter, J. De Vries and H. J. Busscher, Langmuir, 2000, 16, 2845 CrossRef CAS.
- We were limited in these experiments to two sequential low temperature incubation assays because cell lysis became significant at 4 °C, however, the use of high cell concentrations (108 cfu mL−1) and short incubation times gave results with low assay-to-assay variation.
Footnote |
| † Electronic supplementary information (ESI) available: Contact angle goniometry, IR spectroscopy, atomic force microscopy, adhesion force mapping, bioadhesion studies and cell attachment analysis. See http://www.rsc.org/suppdata/jm/b4/b419142k/ |
| This journal is © The Royal Society of Chemistry 2005 |
