Super-hydrophobic/super-hydrophilic patterning of gold surfaces by photocatalytic lithography
Hideo
Notsu
,
Wakana
Kubo
,
Isao
Shitanda
and
Tetsu
Tatsuma
*
Institute of Industrial Science, University of Tokyo, Komaba, Meguro-ku, Tokyo 153-8505, Japan. E-mail: tatsuma@iis.u-tokyo.ac.jp; Fax: +81 3 5452 6338; Tel: +81 3 5452 6336
First published on 14th February 2005
Abstract
Super-hydrophobic and super-hydrophilic gold surfaces were prepared by modifying microstructured gold surfaces with thiols. The perfluorodecanethiol (PFDT)-modified rough gold surface was converted from super-hydrophobic (water contact angle = 150–160°) to super-hydrophilic (0–10°) by photocatalytic remote oxidation using a TiO2 film. During the remote oxidation, oxygen-containing groups were introduced to the thiol, and finally, even sulfur atoms were removed. Super-hydrophobic/super-hydrophilic patterns were also obtained by photocatalytic lithography, by using a TiO2-coated photomask. On the basis of this technique, enzymes and algal cells were patterned on the gold surfaces to fabricate biochips.
Introduction
Patterning of solid surfaces in terms of chemical affinity including hydrophilicity is a technology of importance in the development of various devices and functional materials. In particular, super-hydrophobic/super-hydrophilic patterning would be crucial because it is applicable to the control of liquid flow and the immobilization of functional materials onto specific areas. Potential applications of the patterning may include µ-TAS or lab-on-a-chip devices, micro-chemical reactors, biochips with arrayed DNA, antibodies, enzymes, membrane proteins, cells or organisms, and other sensor arrays.Super-hydrophobic/super-hydrophilic patterns have been developed on microstructured TiO21 and ZnO2 surfaces. However, since the patterning is based on the photocatalytic activity of TiO2 and ZnO, the patterns will be lost under ultraviolet (UV) light, which drives photocatalytic reactions. In addition, these techniques cannot be applied to other materials like metals.
In the present study, we developed a technique to prepare super-hydrophobic/super-hydrophilic patterns on gold surfaces on the basis of photocatalytic lithography. The photocatalytic lithography is a patterning method3,4 that we have recently developed by exploiting photocatalytic remote oxidation.5,6 The patterning is performed by irradiating a solid substrate with UV light thorough a photomask coated with a TiO2 photocatalyst (Fig. 1). In the remote oxidation, aliphatic and aromatic organic compounds are oxidized and decomposed, and even surfaces of diamond, silicon, silver, and copper can be oxidized. Although a similar patterning method that oxidizes polymer or organic thin films by far UV or UV-C without photocatalysis has been reported, such a high energy UV ray is very harmful. On the other hand, only near (or middle) UV is necessary in the photocatalytic lithography. Such a low-energy UV light is much safer and available from more inexpensive light sources. The patterning is possible whether the photocatalyst film and the substrate are in contact or not. Patterning is faster in the contacting mode, while the sample surface is not ready to be damaged in the non-contacting mode. Some other groups have also applied photocatalytic lithography to surface patterning.7,8
 | ||
| Fig. 1 Experimental setups for photocatalytic lithography based on remote oxidation. | ||
The photocatalytic lithography method can be applied to various substrates, and changes between hydrophobic and hydrophilic states are not temporary.3,4 Therefore, the technique should be applicable to the patterning of gold surfaces. Here we selected gold because it is a commonly used electrode material and is used for surface plasmon resonance (SPR) sensors. The patterning by means of removing thiol from the gold surface by UV is also possible. However, in these methods, UV (from a Hg lamp) of stronger power (800 mW cm−2) should be irradiated.9
It is known that the introduction of microscopic roughness to a solid surface enhances hydrophobicity or hydrophilicity of the surface.10 This effect should allow us to prepare super-hydrophobic and super-hydrophilic gold surfaces. We introduced the roughness by electrochemical deposition of gold on the smooth gold surface.11 The roughened surface was modified with a self-assembly monolayer (SAM) of a hydrophobic thiol to obtain a super-hydrophobic surface. A similar technique has been used to make indium tin oxide (ITO) super-hydrophobic.12 Gold half-shells have also been used to prepare super-hydrophobic surfaces.13
Here we also report that super-hydrophilic surfaces can be obtained by coupling the electrochemical deposition of gold with the chemical modification of the gold with thiols having an amino or carboxyl group as the end-group. In addition, when the super-hydrophobic surface modified with the hydrophobic thiol is subjected to photocatalytic remote oxidation, the surface is converted to super-hydrophilic because the hydrophobic groups are oxidized and oxygen-containing groups are introduced. Likewise, super-hydrophobic/super-hydrophilic patterning is also possible by means of photocatalytic lithography. The oxygen-containing groups introduced to the hydrophilic regions can be modified with functional molecules. In the modification process, only the hydrophilic regions are in contact with the solution including the functional molecule, so that the super-hydrophobic regions do not even suffer from adsorption of the molecule.
Experimental
A gold thin film with a smooth surface was deposited on a Pyrex glass plate by sputtering. Gold microstructures were prepared by electrochemical deposition from an aqueous solution containing 40 mM HAuCl4 and 2 mM lead tetraacetate onto the smooth gold surface at −0.08 V vs. Ag|AgCl|KCl(saturated) for 5 min, unless otherwise noted. A Pt wire was used as a counter electrode.The obtained smooth and roughened gold films were immersed into an ethanol solution containing a thiol (10 mM)14 for 1 h to form a SAM on the surfaces. The treatment time was sufficient to convert the roughened surface to super-hydrophobic (see below). Surplus thiol molecules were removed by rinsing with pure ethanol. 2-Aminoethanethiol hydrochloride and 11-mercaptoundecanoic acid (both from Aldrich) were used as thiols with hydrophilic groups. Octanethiol (Aldrich) and 1H,1H,2H,2H-perfluorodecanethiol (Wako Pure Chemical, Japan) were used as hydrophobic thiols.
A TiO2 sol, STS01 (Ishihara Sangyo, Japan) was diluted 1 : 3 with ethanol, sonicated for 30 min, and coated on a non-alkali glass or a non-alkali glass-based photomask (Dai Nippon Printing, Japan) by spin-coating at ∼1500 rpm for 10 s. The TiO2 was calcined at 400 °C for 60 min. Photocatalytic lithography was carried out as shown in Fig. 1. The thiol-modified gold film was faced to the TiO2 coating with 12.5 µm intervening gap by use of polyimide films. The TiO2 coating was irradiated with a Hg–Xe lamp (Luminar Ace, Hayashi Tokei, Japan) with a band-pass filter (300–400 nm, 100 mW cm−2).
A fluorescein isothiocyanate labelled peroxidase (FITC-POD, Sigma) was immobilized onto the oxidized regions of the patterned gold surface. Prior to the immobilization, an aqueous solution containing 0.1% 3-aminopropyltriethoxysilane (APTES) was applied onto the oxidized hydrophilic regions and left for 10 min. After the sample was rinsed with pure water, an aqueous solution containing 0.25 g L−1 FITC-POD and 0.25% glutaraldehyde was applied onto the oxidized regions. The sample was stored in a refrigerator for 2 h, and rinsed with pure water. Immobilization of algae cells Chlorella vulgaris strain NIES-227 (Microbial Culture Collection of National Institute for Environmental Studies, Japan) were performed on the basis of polyion complex formation from poly(L-lysine) (PLL) as a polycation and poly(4-styrenesulfonic acid) (PSS) as a polyanion. First, an aqueous solution containing 107 cells L−1 algae was applied onto the membrane, followed by successive casting of the PLL and PSS solutions.
Results and discussion
Remote oxidation of thiol-modified smooth gold surfaces
Smooth gold surfaces were modified with SAMs of the thiols. Table 1 (second column) lists water contact angles of the thiol-modified and unmodified smooth gold surfaces. Although the bare gold surface was rather hydrophilic, the value of water contact angle increased by the modification with octanethiol (OT) or 1H,1H,2H,2H-perfluorodecanethiol (PFDT). In contrast, the modification with 11-mercaptoundecanoic acid (MUA) or 2-aminoethanethiol (AET) decreased the value of the water contact angle. These results are reasonable because the surface wettability reflects the characteristics of the thiol monolayer.| Water contact angle/°a | ||||
|---|---|---|---|---|
| Smooth gold | Rough gold | |||
| Beforeb | Afterc | Beforeb | Afterc | |
| a Contact angles are average for 3–5 samples. b Before remote oxidation. c After remote oxidation (100 mW cm−2, 30 min). | ||||
| Unmodified | 40–60 | 10–15 | 10–20 | 0–5 |
| OT | 100–110 | 10–20 | 130–150 | 0–10 |
| PFDT | 110–120 | 10–20 | 150–160 | 0–10 |
| MUA | 5–15 | 10–20 | 0–5 | 0–10 |
| AET | 20–40 | 10–20 | 0–5 | 0–10 |
Next, the gold surfaces were subjected to photocatalytic remote oxidation at 100 mW cm−2 for 30 min (Table 1, third column). When the unmodified gold surface was treated, the contact angle value decreased and the surface became more hydrophilic. This may be explained in terms of decomposition of pre-adsorbed organic contaminants on the gold surface and/or oxidation of the gold surface. This is discussed below in the section of analysis.
In the case of the thiol-modified gold surfaces, the final angle was almost the same as that of the treated bare gold surface. These results are suggestive of almost complete removal of the thiols in the remote oxidation. This is reasonable because various organic molecules are decomposed in the remote oxidation.6 The removal was confirmed by X-ray photoelectron spectroscopy (XPS) measurements (described below).
Patterning of smooth gold surfaces
We found that the surface wettability of thiol-modified gold can be changed by the photocatalytic remote oxidation. Next we examined patterning by photocatalytic lithography, by using a TiO2 coated photomask. OT- and MUA-modified smooth gold surfaces were subjected to photocatalytic lithography (100 mW cm−2; 3 h). The obtained patterns were visualized by casting an ethanol solution of rhodamine 6G (125 µM), and observed with a fluorescent microscope (Fig. 2). The oxidized regions exhibited stronger fluorescence than the non-oxidized regions. As can be seen, a pattern with a width of 15 µm could be formed. It is known that the resolution of photoctalytic lithography is better than 5 µm.4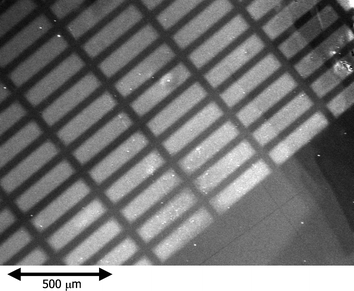 | ||
| Fig. 2 Fluorescent micrograph of the MUA-modified gold surface after photocatalytic lithography (100 mW cm−2, 180 min) and treatment with 125 µM ethanol solution of rhodamine 6G. | ||
Remote oxidation of thiol-modified rough gold surfaces
To emphasize the contrast of wettability, gold microstructures were formed on the smooth gold surface by means of electrodeposition. The obtained rough surfaces were modified with SAMs of the thiols. The water contact angles of the thiol-modified and unmodified rough gold surfaces are also listed in Table 1 (fourth column). In comparison with the data for the smooth gold surfaces (second column), it is obvious that both hydrophilicity (unmodified and MUA- and AET-modified surfaces) and hydrophobicity (OT- and PFDT-modified surfaces) are enhanced. Especially, the PFDT-modified surface exhibited super-hydrophobicity with a water contact angle of 150–160° (Fig. 3a). On the other hand, the MUA- and AET-modified surfaces exhibited super-hydrophilicity with a water contact angle of 0–5°. | ||
| Fig. 3 Water dropped on the (a) non-oxidized and (b) remote oxidized regions of the PFDT-modified rough gold surface. The photocatalytic remote oxidation was performed at 100 mW cm−2 for 10 h. | ||
These results can be explained with the Wenzel equation10 (cosθ = r cosθ′, where θ and θ′ are contact angles on the roughened (roughness factor = r) and completely smooth surfaces, respectively) (in the case of super-hydrophobicity, it may be explained in terms of the Cassie equation15) which indicates that micro-asperity, which was introduced to the present system by the gold electrodeposition, emphasizes both hydrophilicity (water contact angle <90°) and hydrophobicity (contact angle >90°).
The period for the gold deposition has been optimized. In the case of deposition for 1 min, no significant change in the appearance of the gold surface was visually observed. Super-hydrophobicity was not obtained even after the modification with PFDT. As the deposition time exceeded 2 min, the gold surface became orange and metallic lustre disappeared. With increasing treatment time, the color was darker. The size of the microstructures was also increased. In the scanning electron microscopic (SEM) images, 150–250 nm and 400–500 nm of gold structures were observed at the deposition times of 3 and 5 min, respectively (Fig. 4). The roughness factors of the smooth and roughened (deposition time was 5 min) gold surfaces measured by atomic force microscopy (AFM, Seiko Instrument Inc., SPI3800N) were 1.02 and 1.58, respectively. In the case of the electrochemical deposition for 0 and 1 min, the water contact angles after modification with PFDT were 110° and 120°, respectively. In the case of the deposition for 3 min or longer, super-hydrophobicity was obtained after the modification with PFDT. Thus, we prepared the samples for other experiments by 5 min deposition.
 | ||
| Fig. 4 SEM images of deposited gold microstructures. The electrochemical deposition was performed for (a) 3 min or (b) 5 min from an aqueous solution containing 40 mM tetrachloroaurate and 2 mM lead tetraacetate at −0.08 V vs. Ag/AgCl. | ||
The thiol-modified rough gold surfaces were subjected to photocatalytic remote oxidation at 100 mW cm−2 for 30 min (Table 1, fifth column). In all the cases, after the remote oxidation, the surfaces exhibited super-hydrophilicity with a water contact angle of <10° as expected. In the case of PFDT-modified rough gold, the surface was converted from super-hydrophobic (Fig. 3a) to super-hydrophilic (Fig. 3b). However, no significant change in the contact angle value was observed during irradiation with UV of the same intensity without TiO2 photocatalyst for at least 10 h.
Analysis of the thiol decomposition
XPS measurements were performed in order to understand how thiols were oxidized and decomposed. In the XPS spectra of the unmodified gold surfaces (both smooth and roughened), C 1s and O 1s peaks, which may be ascribed to the pre-adsorbed organic contaminants, were observed. After photocatalytic remote oxidation for 3 h, the C 1s peak was weakened, suggesting that the contaminants were decomposed. The O 1s peak was enhanced and shifted to lower binding energy after the remote oxidation. This is indicative of the generation of a metal oxide. Moreover, in the case of Au 4f peaks, the shoulders appeared at higher binding energy after the remote oxidation (Fig. 5). These shoulders, which have been observed for electrochemically generated gold oxide layers,16 correspond to gold atoms bound to atoms that have high electronegativity. On the basis of these results, we conclude that the gold surfaces can be oxidized to gold oxide by the photocatalytic remote oxidation, as well as silver and copper.3,4 | ||
| Fig. 5 Au 4f core level XPS spectra of an unmodified smooth gold surface before (solid curve) and after (dashed curve) the photocatalytic remote oxidation (spacer thickness, 12.5 µm; light intensity, 100 mW cm−2; treatment time, 10 h). Similar results were also obtained for a roughened gold surface. | ||
In the XPS spectra of OT-modified gold surfaces (both smooth and roughened), a strong C 1s peak due to OT was observed. On the other hand, an O 1s peak was not observed, suggesting that there was no adsorbed water because the surface was highly hydrophobic. An S 2p peak due to OT and Au 4f peaks due to the gold under the SAM layer were also observed. Time courses of the intensities of Au 4f, C 1s, O 1s, and S 2p peaks during the photocatalytic remote oxidation are shown in Fig. 6, together with those of the water contact angle.
 | ||
| Fig. 6 Time courses of (a) water contact angle and (b) area of core level XPS peaks of the OT-modified gold surface. The photocatalytic remote oxidation was performed at 100 mW cm−2. | ||
As with the C 1s peak, the intensity decreased gradually, indicating the decomposition of the alkyl group of OT. The increase in the intensity of the O 1p peak may reflect the generation of oxygen-containing groups (e.g., –OH, >CO, and –COOH) during the decomposition of OT. The increase observed for the Au 4f peak intensity corresponds to the removal of the alkyl group, which has partially shielded Au from the detection. The S 2p peak mainly located at 163 eV, corresponding to the mercapto group, was observed before the treatment and its intensity decreased gradually during the remote oxidation. Simultaneously, a new S 2p peak was formed at 168 eV and grew up during the oxidation. The latter peak may be assigned to an oxidized sulfur, probably R–SO3H or SO2.17 After the treated sample, which was not fully oxidized, was rinsed with pure water, the peak at 163 eV was unchanged, but that at 168 eV disappeared, because small R–SO3H molecules and SO2 should be water-soluble. Thus, we conclude that the Au–S bonding can be cleaved by the remote oxidation.
Similar results were also obtained for PFDT-modified gold. In this case, a strong F 1s peak was suppressed gradually by photocatalytic oxidation, and completely vanished in about 60 min. This suggests that PFDT is decomposed to some small molecules that are volatilized during the photocatalytic remote oxidation or during the XPS measurement.
The time courses of the water contact angle indicate that the decomposition of all OT (or PFDT) was not necessary for obtaining the super-hydrophilic surface. The oxygen-containing groups generated in the course of the decomposition should have contributed to the increase in the hydrophilicity.
One of the drawbacks of the present patterning method is unknown surface composition of the oxygen-containing groups. Nevertheless, the patterning on the basis of the wettability is possible, and functional molecules can be introduced to the surface via the oxygen-containing groups.
Patterning of enzymes and cells
Patterning of enzymes on a gold surface was examined on the basis of the super-hydrophobic/super-hydrophilic patterns. The PFDT-modified rough gold surface was patterned by means of photocatalytic lithography for 30 min. It is obvious from Fig. 6 that the treatment for 30 min is sufficient to convert the irradiated region to super-hydrophilic and to introduce oxygen-containing groups to the SAM. The labelled peroxidase (FITC-POD) was immobilized on the surface of the irradiated region, via the oxygen-containing groups, APTES, and glutaraldehyde. The obtained enzyme pattern was observed by a fluorescent microscope (Fig. 7a). | ||
| Fig. 7 Fluorescent micrographs of (a) the gold surface patterned with FITC-POD and (b) the gold surface patterned with algal cells. A PFDT-modified rough gold surface was subjected to photocatalytic lithography (100 mW cm−2, 60 min), and the irradiated regions were modified with FITC-POD or the cells. | ||
Living algal cells were also patterned on the gold surface. Most algal cells were successfully immobilized onto the hydrophilic area of the pattern prepared by photocatalytic lithography (Fig. 7b). It was clearly confirmed that the enzyme and algae were immobilized only in the area in which photocatalytic remote oxidation was performed.
Acknowledgements
The authors are grateful to Dai Nippon Printing for the supply of photomasks. This study was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (Area No. 417, Research No. 14050028 for TT) and a Grant-in-Aid for Encouragement of Young Scientists (for HN) both from the Ministry of Education, Culture, Sports, Science and Technology of Japan.References
- K. Tadanaga, J. Morinaga, A. Matsuda and T. Minami, Chem. Mater., 2000, 12, 590 CrossRef CAS.
- X.-T. Zhang, O. Sato and A. Fujishima, Langmuir, 2004, 20, 6065 CrossRef CAS.
- T. Tatsuma, W. Kubo and A. Fujishima, Langmuir, 2002, 18, 9632 CrossRef CAS.
- W. Kubo, T. Tatsuma, A. Fujishima and H. Kobayashi, J. Phys. Chem. B, 2004, 108, 3005 CrossRef CAS.
- T. Tatsuma, S. Tachibana, T. Miwa, D. A. Tryk and A. Fujishima, J. Phys. Chem. B, 1999, 103, 8033 CrossRef CAS.
- T. Tatsuma, S. Tachibana and A. Fujishima, J. Phys. Chem. B, 2001, 105, 6987 CrossRef CAS.
- Y. Ishikawa, Y. Matsumoto, Y. Nishida, S. Taniguchi and J. Watanabe, J. Am. Chem. Soc., 2003, 125, 6558 CrossRef CAS.
- J. P. Lee and M. M. Sung, J. Am. Chem. Soc., 2004, 126, 28 CrossRef CAS.
- M. Ohtani, T. Sunagawa, S. Kuwabata and H. Yoneyama, J. Electroanal. Chem., 1995, 396, 97 CrossRef CAS.
- R. N. Wenzel, J. Phys. Colloid Chem., 1949, 53, 1466 CAS.
- S. Toyama, M. Someya, O. Takei, T. Ohtake, R. Usami, K. Horikoshi and Y. Ikariyama, Chem. Lett., 2001, 160 CrossRef CAS.
- X. Zhang, F. Shi, X. Yu, H. Liu, Y. Fu, Z. Wang, L. Jiang and X. Li, J. Am. Chem. Soc., 2004, 126, 3064 CrossRef CAS.
- J. C. Love, B. D. Gates, D. B. Wolfe, K. E. Paul and G. M. Whitesides, Nano Lett., 2002, 2, 891 CrossRef CAS.
- H. Rieley, G. K. Kendall, F. W. Zemicael, T. L. Smith and S. Yang, Langmuir, 1998, 14, 5147 CrossRef CAS.
- A. B. D. Cassie, Discuss. Faraday Soc., 1948, 3, 11 RSC.
- K. Juodkazis, J. Juodkazyte, V. Jasulaitiene, A. Lukinskas and B. Sebeka, Electrochem. Commun., 2000, 2, 503 CrossRef CAS.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, in Handbook of X-ray Photoelectron Spectroscopy, ed. J. Chastain and R. C. King, Jr., Physical Electronics, Eden Prairie, MN, 1995 Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |
