Restriction of CaCO3 polymorph by NH⋯O hydrogen-bonded poly(methacryloylaminocarboxylate) ligands: induced polymorph change by strength and/or formation manner of hydrogen bond†
Kazuyuki
Takahashi
a,
Atsuko
Kobayashi
b,
Mototsugu
Doi
a,
Seiji
Adachi
a,
Takahisa
Taguchi
b,
Taka-aki
Okamura
a,
Hitoshi
Yamamoto
a and
Norikazu
Ueyama
*a
aDepartment of Macromolecular Science, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan. E-mail: ueyama@chem.sci.osaka-u.ac.jp; Fax: +81-6-6850-5474; Tel: +81-6-6850-5449
bSpecial Section of Human Life Technology, National Institute of AIST Kansai, Ikeda, Osaka 563-8577, Japan
First published on 4th March 2005
Abstract
Novel poly(methacryloylaminocarboxylate) ligands were synthesized as models for an acidic peptide in CaCO3 biominerals. Syndiotactic poly[3-methyl-2-(methacryloylamino)butyrate], isotactic-rich poly[3-(methacryloylamino)propionate], and syndiotactic poly[4-(methacryloylamino)butyrate] form 5-, 6-, and 7-membered-ring intra-side-chain NH⋯O hydrogen bonds, respectively, between a carboxylate oxyanion and the neighboring amide NH proton, while syndiotactic poly[3-(methacryloylamino)propionate] forms intermolecular NH⋯O hydrogen bonds. These polymer ligands strongly bind to CaCO3 crystals because of the NH⋯O hydrogen bonds. In polymer ligand–CaCO3 composites, the strength and/or mode of hydrogen bonding in the polymer ligands affects the CaCO3 polymorphs. Five-membered-ring intra-side-chain and intermolecular hydrogen-bonded polymers have weak hydrogen bonds and produce calcite crystals, whereas other ligands with 6- and 7-membered-ring intra-side-chain hydrogen bonds have strong hydrogen bonds and yield vaterite crystals. Polymorph change of CaCO3 crystal is induced by differences of strength and/or mode of the NH⋯O hydrogen bond.
Introduction
Biominerals occurring in nature, such as those that make up mollusk shells, teeth, and magnetotactic bacterial magnetosomes, are usually composed of biopolymers and inorganic crystals, e.g., CaCO3, Ca10(PO4)6(OH)2, Fe3O4, etc.1 These biomineralization processes are often mediated and regulated by a small amount of a biopolymer ligand.2 When shells biomineralize, for example, oriented CaCO3 crystals grow on a nucleating protein sheet.3–6 Through this process, a small amount of organic material binds to the crystal face and controls the crystal growth, and the resulting biominerals possess highly controlled shapes and sizes under ambient conditions.6 Actually, our recent report indicates that each aragonite brick in the nacreous layer of Pinctada fucata (Japanese pearl oyster) is not a single crystal but a composition of nano-ordered aragonite crystals and that biopolymers participate in the generation and orientation of aragonite nanocrystals.7In CaCO3 architectures, unusually acidic proteins are found in aragonite- (stable form of CaCO3) or calcite- (the most stable form of CaCO3) containing mineralized tissues of invertebrates, which are rich in Asx (i.e., Asp or Asn) and/or Glx (i.e., Glu or Gln).8–11 For example, the soluble protein from the mollusk Atrina rigida contains about 40 mol% of acidic residues (Asx and Glx).10 The protein components of the biopolymer ligands from the ascidian spicules, which are extracted from the amorphous CaCO3 produced by sponges,9 are rich in Glu and/or Gln.11 Interestingly, Falini et al. demonstrated that when the assemblage of unusually acidic proteins from an aragonite layer is added, aragonite crystal forms, and when the proteins are derived from a calcite layer, calcite crystal forms.12 Acidic residues of most of the proteins affect the mineralization of CaCO3 architectures.
Artificial carboxylate or poly(carboxylate) ligand–CaCO3 composites were used as models for biominerals that contain acidic peptides in the previous reports.5,13–18 Some reports treated unique ligands having a extremely elaborate structure, such as anionic porphyrins,14 dendrimers,15 helical poly(isocyanide),16 calixarenes,17 aerosol thin films,18etc. It has been shown that the carboxylate produces a specific surface during CaCO3 crystallization.
The strong binding of biopolymer ligands to a CaCO3 crystal is one of the puzzles of mimicking biominerals. In this respect, we have discussed the scenario of an NH⋯O hydrogen bonding between a Ca(II)-coordinated oxygen atom and the neighboring amide NH proton. This NH⋯O hydrogen bond prevents the metal–oxygen bond from dissociating19–21 like the NH⋯S hydrogen bond, which shifts the pKa value of a thiol22 and stabilizes the metal–thiolate complexes.23,24 The cytochrome P-450 model (porphinato)(thiolato)iron(III) complexes with single or double NH⋯S hydrogen bonds are stable against O2 or H2O,23 although all the known iron(III) porphyrin thiolato complexes have been reported to be unstable in the presence of air and moisture.25 Furthermore, 2,6-diacetylaminobenzoate has a higher formation constant toward Ca2+ ion than does nonsubstituted benzoate because of the formation of an intramolecular NH⋯O hydrogen bond.21 In biominerals that contain acidic peptides, these effects of NH⋯O hydrogen bonds probably contribute to the stabilization of a Ca–carboxylate oxygen bond.
Previously, we synthesized poly(carboxylate) ligands that had an NH⋯O hydrogen bond between a carboxylate oxygen and the neighboring amide NH proton; we used this construct as an artificial model for acidic peptides in CaCO3 biominerals.26–28 These polymer ligands strongly bind to CaCO3 crystals through the hydrogen bond. In the absence of such a hydrogen bond, the metal–oxygen bond is readily hydrolyzed under neutral conditions. Addadi et al. also reported the difference between poly(acrylate) and native acidic peptides.5 However, our polymer ligands have problems in terms of stereoselectivity or conformation of a polymer main-chain, because these polymers were prepared by polymer reactions that include poly(carboxylic anhydride) with alkylamine,26,27 or poly(allylamine) with carboxylic anhydride.28 Additionally, introduction of a hydrogen-bonded unit to a polymer side-chain was not in so high yield.
In the present study, we synthesized novel poly(methacryloylaminocarboxylate) ligands having 5-, 6-, or 7-membered-ring intra-side-chain NH⋯O hydrogen bonds (Chart 1). These polymer ligands were prepared from the corresponding monomer units and the stereochemistry of the polymer main-chain was controllable. Furthermore, all side-chains contain a hydrogen-bonded unit. In this paper, we demonstrate that these polymer ligands restrict CaCO3 morphologies by utilizing 13C cross-polarization/magic angle spinning (CP/MAS) NMR, X-ray powder diffraction (XRD), and field emission/scanning electron microscopy (FE/SEM) techniques.
 | ||
| Chart 1 | ||
Experimental
Materials
Methacryloyl chloride was obtained from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan) and distilled before use. Synthetic procedures of monomers, MA-Val-OPac, MA-β-Ala-OBzl, and MA-γ-Abu-OtBu, are described in the ESI.† Other reagents were commercially obtained and solvents were used after distillation. All synthetic procedures were performed under Ar atmosphere.Poly(MA-Val-OPac)
A mixture of MA-Val-OPac (761 mg, 2.5 mmol) and 2,2′-azobisisobutyronitrile (AIBN) (3.2 mg, 20 µmol) was stirred at 353 K. After 2 days, 2 mL of CH2Cl2 was added into the mixture and the solution was added dropwise into n-hexane. The precipitate was collected by filtration and dissolved into 2 mL of CH2Cl2. The solution was added dropwise into MeOH to give a white powder. The white powder was collected by filtration and dried over P2O5 under reduced pressure. Yield, 21%. Mn = 54000 Da. Mw/Mn = 1.29.Poly(MA-Val-OH) (1H)
To poly(MA-Val-OPac) (160 mg, 530 µmol) in 20 mL of AcOH was added Zn powder (10.0 g) and the mixture was stirred at room temperature. After 4 hours, the solution was filtered to remove Zn powder and filtrate was concentrated. The residue was washed with CH2Cl2 and 2% HCl aqueous solution. Yield, 45 mg (46%). Stereoselectivity (diad): isotactic/syndiotactic = 23/77. The deprotection of carboxyl groups in the polymer side-chain proceeded in 50% yield from 1H NMR spectra.Poly(MA-β-Ala-OBzl)
A mixture of MA-β-Ala-OBzl (1.0 g, 4.0 mmol) and benzoyl peroxide (BPO) (9.8 mg, 40 µmol) was stirred at 353 K. After one day, 10 mL of CH2Cl2 was added into the mixture and the solution was added dropwise into n-hexane to give a white powder. The white powder was collected by filtration and dried over P2O5 under reduced pressure. Yield, 690 mg (69%). Mn = 3100 Da. Mw/Mn = 1.48.Poly(MA-β-Ala-OH) (2H)
To poly(MA-β-Ala-OBzl) (1.32 g, 5.3 mmol) in 10 mL of MeOH was added 1 M NaOH aqueous solution (5.3 mL, 5.3 mmol) and the solution was stirred at room temperature. After 12 hours, the solution was concentrated and to the residue was added excess 3.5% HCl aqueous solution. The solution was concentrated to dryness. The residue was dissolved in 10 mL of MeOH and the solution was added dropwise into 100 mL of ether to give a white powder. The white powder was collected by centrifugation and dried over P2O5 under reduced pressure. Yield, 498 mg (59%). Stereoselectivity (diad): isotactic/syndiotactic = 16/84.Isotactic-rich poly(MA-β-Ala-OBzl)
A mixture of MA-β-Ala-OBzl (2.2 g, 9.0 mmol), AIBN (15 mg, 90 µmol) and Y(OTf)3 (480 mg, 0.90 mmol) in 2 mL of MeOH was stirred at 333 K. After 8 hours, 50 mL of CH2Cl2 was added into the mixture and the solution was added dropwise into n-hexane (1 L) to give a white powder. The white powder was collected by filtration and dried over P2O5 under reduced pressure. Yield, 2.1 g (95%). Mn = 910. Mw/Mn = 1.03.Isotactic-rich poly(MA-β-Ala-OH) (2isoH)
2isoH was obtained by hydrolysis of the isotactic-rich poly(MA-β-Ala-OBzl). This procedure was the same as the method for the hydrolysis of syndiotactic poly(MA-β-Ala-OBzl). Stereoselectivity (diad): isotactic/syndiotactic = 69/31.Poly(MA-γ-Abu-OtBu)
A mixture of MA-γ-Abu-OtBu (830 mg, 3.7 mmol) and AIBN (6.0 mg, 37 µmol) was stirred at 353 K. After one day, 5 mL of CH2Cl2 was added into the mixture and the solution was added dropwise into n-hexane to give a white powder. The white powder was collected by filtration and dried over P2O5 under reduced pressure. Yield, 378 mg (46%). Mn = 1700 Da. Mw/Mn = 1.19.Poly(MA-γ-Abu-OH) (3H)
Poly(MA-γ-Abu-OtBu) (1.235 g, 5.4 mmol) was dissolved into 10 mL of THF at 353 K. A mixture of NaOH (450 mg, 11 mmol) aqueous solution (2 mL) and 3 mL of EtOH were added to the solution. The solution was stirred at 353 K for one day and then THF and EtOH were removed under reduced pressure. The residue was suspended in water and the solution was stirred at room temperature. After one day, 3.5% HCl aqueous solution was added to the solution to give a white powder. Yield, 664 mg (71%). Stereoselectivity (diad): isotactic/syndiotactic = 22/78.Crystallization of CaCO3 composites in the presence of polymer ligands
Ten mL of an aqueous calcium chloride solution (1 M) was added to 10 mL of a methanol solution of a polymer ligand (10 mM per repeated unit). After stirring for 3 hours, 10 mL of an aqueous ammonium carbonate solution (1 M) were dropped into the mixed solution and then it was stirred for 3 hours. CaCO3 crystallization was carried out by mixing these solutions at 303 K under acidic conditions (pH 3.8–4.0). CaCO3–polymer composites were obtained at pH 7.2–7.4. The final CaCO3/polymer ligand ratio was 100/1. The obtained CaCO3 crystals were washed with methanol and distilled water to remove unbound polymer ligand, and dried over P2O5 under reduced pressure.Physical measurements
Solution-state Fourier transform infrared (FT-IR) spectra in chloroform-d were obtained with a Jasco FT-IR 8300. 400 MHz 1H NMR spectra were taken on a JEOL JNM-GSX400 in dimethyl sulfoxide-d6 or chloroform-d, at 303 K. Tetramethylsilane was used as a standard on 1H NMR measurements. 500 MHz 1H NMR and 1D TOCSY spectra were recorded by a JEOL JNM-LA500 in chloroform-d at 303 K. 75 MHz solid-state 13C CP/MAS spectra were recorded on a Chemagnetics CMX-300 spectrometer at room temperature. The chemical shifts of CP/MAS spectra were calculated from the methyl group of hexamethylbenzene (17.36 ppm). The rotating speed was 4000 rps, and contact time was 1.00 ms in the CP/MAS measurements. Gel permeation chromatographic (GPC) analyses were carried out at 313 K using Tosoh MultiporeHXL-M columns connected to a Tosoh UV detector and RI detector. THF was used as an eluent at a flow rate of 0.8 mL min−1. Samples were dissolved in THF (1.0 mg mL−1) and were filtered in order to remove particulates before injection. The molecular weight was calibrated according to poly(styrene) standards. FE/SEM images were obtained with a Hitachi S-5000 at 20 kV acceleration voltages. Samples for FE/SEM analysis were vapor deposited with osmium oxide. XRD patterns were taken on a Rigaku RAD system at room temperature. The radiation used was CuKα monochromatized with graphite.Results
Polymer ligand preparation
The three types of polymer ligands, poly[3-methyl-2-(methacryloylamino)butylic acid] (1H), poly[3-(methacryloylamino)propionic acid] (2H), and poly[4-(methacryloylamino)butyric acid] (3H), were synthesized by the reactions as shown in Scheme 1. The monomer preparations for 1H, 2H, and 3H were performed by the reaction of methacryloyl chloride with the corresponding C-terminal-protected amino acids, H-Val-OPac, H-β-Ala-OBzl, and H-γ-Abu-OtBu, respectively (see ESI†). The polymerizations were carried out by using AIBN or BPO to induce free radical polymerization. After the polymerization, the carboxylic acid group in a polymer side-chain was treated by a typical deprotection procedure. The synthesis of these polymer ligands was confirmed by 1H NMR spectra. Fig. 1 shows the 1H NMR spectra of the polymer ligands in Me2SO-d6 at 303 K. Amide NH 1H NMR signals in these polymer ligands were observed at 8.00, 7.40, and 7.28 ppm, for 1H, 2H, and 3H, respectively. The 1H NMR signal of the COOH group in each polymer ligand appeared at levels between 12 and 13 ppm. Other 1H NMR signals of the polymer main-chain and the side-chain were also observed at a high magnetic field above 4.0 ppm (detailed signal assignments are described in the ESI†). | ||
| Fig. 1 1H NMR spectra of (a) 1H, (b) 1NMe4, (c) 2H, (d) 2NMe4, (e) 2isoH, (f) 2isoNMe4, (g) 3H and (h) 3NEt4 (303 K, Me2SO-d6). | ||
Polymer ligands 2 and 3 completely undergo the deprotection procedure, while the conversion of 1 was 50% from the 1H NMR spectrum. The remaining C-terminal-protected group, the ester group, does not affect the crystallization of polymer ligand–CaCO3 composites, because the formation constant of the ester group toward the Ca2+ ion is lower than that of the carboxylate group. Additionally, the ester group is not able to neutralize the charge of the Ca2+ ion on the surface of CaCO3 crystals.
The number-average molecular weight (Mn) of each polymer ligand was determined by GPC in THF solution (Table 1). The Mn value of the polymer ligand was obtained in the COOR form, because the polymer ligands in the COOH form associate with each other in THF solution. The obtained Mn values of these polymer ligands were 54000, 3100, and 1700 Da, for 1, 2, and 3, respectively. Interestingly, the polydispersities of the polymer ligands appear in a relatively narrow range (Mw/Mn = 1.19 to 1.48), whereas similar amino acid-containing polymers obtained by free radical polymerization have a wide range of values as reported previously.29
| M n a | M w/Mna | mm | mr + rm | rr | m | r | |
|---|---|---|---|---|---|---|---|
| a Determined by GPC (THF, PS standard) with ester form of polymer. b Estimated with ester form by 13C NMR spectra in chloroform-d at 303 K. c Estimated with COOH form by 13C NMR spectra in Me2SO-d6 at 353 K. | |||||||
| 1 | 54000 | 1.29 | 5b | 37b | 58b | 23b | 77b |
| 2 | 3100 | 1.48 | 0c | 33c | 67c | 16c | 84c |
| 2iso | 910 | 1.03 | 37c | 63c | 0c | 69c | 31c |
| 3 | 1700 | 1.19 | 3c | 38c | 59c | 22c | 78c |
Polymer tacticity was determined utilizing a 13C NMR measurement in Me2SO-d6 at 353 K as listed in Table 1. Estimation for tactic triad, that is meso–meso (mm), racemo–racemo (rr), meso–racemo (mr), or racemo–meso (rm), was performed based on quaternary carbon signals of the polymer ligand.30 Generally, a stereospecific polymer is rarely obtained by a radical polymerization and only atactic or slightly syndiotactic-rich polymer was produced.31 In our case, the results indicate that polymer ligands 1, 2, and 3 hardly form the mm triad. The obtained values of syndiotactic diad content are 77, 84, and 78% for 1, 2, and 3, respectively. Therefore, the polymer ligands have a highly controlled syndiotactic tacticity with a narrow polydispersity.
Isotactic-rich poly[3-(methacryloylamino)propionic acid] (2isoH) was synthesized in order to examine the effects of the polymer tacticity on CaCO3 crystallization. Preparation of isotactic-rich polymer was performed by free radical polymerization with a Lewis acid (Scheme 1). We obtained the syndiotactic polymers (1H, 2H, and 3H) without the Lewis acid. Recently, several reports described the synthesis of stereocontrolled polymer by using various Lewis acids or amide NH-protected monomer.32,33 Because acrylamide and methacrylamide monomers easily undergo proton-transfer polymerizations in an anionic polymerization, that is usually used in order to obtain an isotactic polymer. For example, a Lewis acid, Y(OTf)3, was employed as a catalyst for a stereospecific radical polymerization in order to obtain an isotactic-rich polymer. Use of the AIBN–Y(OTf)3–MeOH system for radical polymerization controls thestereoregularity, and the obtained polymer has a high isotactic content (80%) at mild temperature (333 K).33 In the present study, 2isoH includes isotactic content, which is about 70% on the basis of the 13C NMR spectral data, and has a narrow polydispersity (Mw/Mn = 1.03) (Table 1).
Formation of an NH⋯O hydrogen bond in polymer ligands
Formation of an NH⋯O hydrogen bond in the polymer side-chain was examined with simple model ligands, i.e., 3-methyl-2-(2,2,2-triphenylacetylamino)-butyrate (4), 3-(2,2,2-triphenylacetylamino)-propionate (5), and 4-(2,2,2-triphenylacetylamino)-butyrate (6) (Chart 2), for polymer-repeated units of 1, 2, and 3, respectively. These model ligands have a bulky triphenylacetylamino group to avoid polynuclear complexation and will be discussed in detail. The 1H NMR spectra of these model ligands in chloroform-d are shown in ESI† Fig. S1a–f. In the carboxylic acid form, the amide NH 1H NMR signals of 3-methyl-2-(2,2,2-triphenylacetylamino)-butyric acid (4H), 3-(2,2,2-triphenylacetylamino)-propionic acid (5H), and 4-(2,2,2-triphenylacetylamino)-butyric acid (6H) were observed at 6.22, 6.31, and 5.96 ppm, respectively. On the other hand, the amide NH 1H NMR signals of tetramethylammonium carboxylate of 4 (4NMe4), 5 (5NMe4), and 6 (6NMe4) were observed at 6.64, 7.07, and 6.13 ppm with downfield shifts of 0.42, 0.76, and 0.17 ppm, respectively, from those of the corresponding carboxylic acid. Thus, relatively downfield shifts of amide NH 1H NMR signals indicate the formation of an intramolecular NH⋯O hydrogen bond. | ||
| Chart 2 | ||
The temperature coefficient was obtained by the variable-temperature (243 to 303 K) measurements of 1H NMR spectra in chloroform-d, to confirm the intramolecular hydrogen bonding. The temperature coefficients of the amide NH signals of 4H, 4NMe4, 5H, 5NMe4, 6H, and 6NMe4 are −0.37, −1.2, −2.7, −1.2, −2.2, and −1.7 ppb K−1, respectively. These low coefficients of the carboxylate anions (4NMe4, 5NMe4, and 6NMe4) indicate that an intramolecular NH⋯O hydrogen bond forms in each carboxylate anion. In general, the formation of a stable intramolecular NH⋯O hydrogen bond in linear peptides gives a more positive temperature coefficient than the value of −2.4 ppb K−1 found in chloroform-d.34 The model ligands for polymer ligands also have a more positive temperature coefficient than the value in the corresponding carboxylic acid. Thus, these models form the intramolecular NH⋯O hydrogen bond.
Intramolecular or intermolecular hydrogen bond formation was also established by a FT-IR measurement in chloroform-d (Fig. 2). The amide NH IR band of 4NMe4 was observed at 3377 cm−1, although that of 4H was observed at 3455 cm−1. Similar low wavenumber shifts were found in the other carboxylate anions, 5NMe4 and 6NMe4, by 418 and 424 cm−1, respectively. The large low-wavenumber shifts in the amide NH band between the carboxylic acid and the carboxylate anion forms indicate the formation of the intramolecular NH⋯O hydrogen bonds. The degree of shifts of the amide NH bands in the FT-IR corresponds to the strength of the NH⋯O hydrogen bond.20,35 Therefore, 5NMe4 and 6NMe4 form strong NH⋯O hydrogen bonds, whereas 4NMe4 forms a weak NH⋯O hydrogen bond. These amide NH IR bands do not shift in dilute solution (2 mM solution concentration), which excludes the possibility of formation of intermolecular NH⋯O hydrogen bonds. These results for 4, 5, and 6 in chloroform-d demonstrate that each model ligand forms an intramolecular NH⋯O hydrogen bond as shown in Chart 2.
 | ||
| Fig. 2 FT-IR spectra in 10 mM chloroform solution of (a) 4H, (b) 4NMe4, (c) 5H, (d) 5NMe4, (e) 6H and (f) 6NMe4. The assignments of NH IR bands of 5NMe4 and 6NMe4 were determined from deuterium derivatives 7 and 8, deuterated 5NMe4 and 6NMe4, respectively (detailed synthetic procedures are shown in ESI†). The ND vibration bands for 7 and 8 are at 2199 cm−1 and 2197 cm−1, respectively. | ||
In a polar solvent, such as Me2SO-d6, these model ligands form the intramolecular NH⋯O hydrogen bond similar to those observed in the less polar solvent, chloroform-d. The amide NH 1H NMR signals of 4, 4NMe4, 5, 5NMe4, 6, and 6NMe4 in Me2SO-d6 appear at 6.42, 6.94, 7.10, 8.08, 7.17, and 7.19 ppm, respectively (ESI† Fig. S1g-l). The amide NH 1H NMR signals of these model ligands in the carboxylate anion form also show downfield shifts of 0.52, 0.98, and 0.02 ppm for 4NMe4, 5NMe4, and 6NMe4, respectively, compared with those in the carboxylic acid form. Thus, these results indicate that the model ligands form the intramolecular NH⋯O hydrogen bond in both the polar and less polar solvents.
In polymer ligands, 2NMe4 exhibits a different trend in the 1H NMR spectra (Fig. 1) compared with the others, although all polymer ligands, 1NMe4, 2NMe4, 2isoNMe4, and 3NEt4, are suggested to form intra-side-chain NH⋯O hydrogen bonds from the results of the model ligands. The amide NH 1H NMR signal of 2NMe4 appears at 7.38 ppm with upfield shifts of 0.02 ppm (Fig. 1d), while the signals of 1NMe4, 2isoNMe4, and 3NEt4 are observed with 1.50, 1.40, and 1.02 ppm downfield shifts, respectively (Fig. 1: b, f, and h). Additionally, the amide NH 1H NMR signal of 2NMe4 is relatively broad. This result gives the possibility of intermolecular NH⋯O hydrogen bonds in 2NMe4. Previously, we have demonstrated that an amide NH 1H NMR signal of intermolecular NH⋯S hydrogen-bonded tetrapeptide was broadened by formation of an intermolecular hydrogen-bonded polymeric structure.36 In this case, 2NMe4 also forms intermolecular NH⋯O hydrogen bonds. In contrast, other polymer ligands, 1NMe4, 2isoNMe4, and 3NEt4, cannot form such intermolecular interactions, because their side-chains hinder the formation of intermolecular hydrogen bonds. Thus, three polymer ligands, 1NMe4, 2isoNMe4, and 3NEt4, form intra-side-chain NH⋯O hydrogen bonds as well as these models. In contrast, syndiotactic-rich 2NMe4 forms intermolecular NH⋯O hydrogen bonds, as shown in Fig. 3. However, isotactic-rich 2isoNMe4 allows the formation of intra-side-chain NH⋯O hydrogen bonds even in the solid-state. This possibility is discussed later.
 | ||
| Fig. 3 Schematic drawings of hydrogen-bonded polymers: (a) 2NMe4 and (b) 2isoNMe4. | ||
Observation of polymer ligands in CaCO3 composites by 13C CP/MAS NMR
To observe the polymer ligands in the CaCO3 composites, we measured the 13C CP/MAS spectra of polymer ligand 1–CaCO3 composite (1CaCO3), 2–CaCO3 composite (2CaCO3), 2iso–CaCO3 composite (2isoCaCO3), and 3–CaCO3 composite (3CaCO3) (Fig. 4) and compared these with the spectra of these poly(carboxylic acid) and poly(carboxylate) anion without CaCO3 (Fig. 5). The 13C CP/MAS NMR measurement is useful to obtain chemical information of the polymer ligand in a CaCO3 composite.7,37,38 The chemical shift of each 13C signal of the carbonyl carbons was estimated by using curve-fitting analysis. The 13C signals of CONH and COOH of 1H are observed at 177 and 180 ppm, respectively (Fig. 5a), and those signals for 3H also appear at the same chemical shifts for 1H (Fig. 5g). The 13C signals of CONH and COOH of 2H are observed at 174 and 179 ppm, respectively (Fig. 5c), and those in 2isoH appear at 175 and 178 for CONH and COOH, respectively (Fig. 5e). On the other hand, the 13C signals of COO− of 1NMe4, 2NMe4, 2isoNMe4, and 3NEt4 show downfield shifts by 1, 2, 2, and 2 ppm, respectively (Fig. 5: b, d, f, and h), from those of the corresponding poly(carboxylic acid)s. The 13C signals of CONH of 2NMe4, 2isoNMe4, and 3NEt4 also show downfield shifts of 5, 2, and 2 ppm, respectively (Fig. 5: d, f, and j). These results indicate that environments around the carbonyl carbon in the polymer ligands change between the carboxylic acid and the carboxylate anion forms. | ||
| Fig. 4 Solid-state 13C CP/MAS spectra of (a) 1CaCO3, (b) decombined analysis of carbonyl region of 1CaCO3, (c) 2CaCO3, (d) decombined analysis of carbonyl region of 2CaCO3, (e) 2isoCaCO3, (f) decombined analysis of carbonyl region of 2isoCaCO3, (g) 3CaCO3 and (h) decombined analysis of carbonyl region of 3CaCO3 (where: * is a spinning side band; # is a background peak). | ||
 | ||
| Fig. 5 Solid-state 13C CP/MAS spectra of (a) 1H, (b) 1NMe4, (c) 2H, (d) 2NMe4, (e) 2isoH, (f) 2isoNMe4, (g) 3H, and (h) 3NEt4 (where: * is a spinning side band). | ||
In the polymer ligand–CaCO3 composites, the 13C NMR signals of the polymer ligands appear near the carbonate 13C signal (13CO32−) in 13C CP/MAS spectra (Fig. 4). The curve-fitting shows the 13C signals of CONH and COO− of 1CaCO3 are at 179 and 184 ppm, respectively (Fig. 4b). The 13C signals of CONH and COO− of 2CaCO3 appear at 175 and 181 ppm, respectively (Fig. 4d), and those of CONH and COO− of 2isoCaCO3 are also observed at 173 and 181 ppm, respectively (Fig. 4f). The 13C signals of CONH and COO− of 3CaCO3 appear at 177 and 183 ppm, respectively (Fig. 4h). The observation of these 13C CP/MAS signals indicates that the polymer ligand in CaCO3 composites remained after washing. No 13C signal of a polymer ligand should be observed in the 13C CP/MAS spectrum if the polymer ligand is easily removed from the CaCO3 crystals upon washing.38 Thus, these polymer ligands still bind to CaCO3 crystals as the poly(carboxylate) anion form after the washing process.
Morphologies of poly(carboxylate) ligand–CaCO3 composites
Morphologies of polymer ligand–CaCO3 composites were estimated via XRD measurements (Fig. 6). It is known that the main morphologies of CaCO3 crystal are calcite, vaterite, and aragonite.1 Our polymer ligand–CaCO3 composites did not contain aragonite, as was confirmed by XRD observation. The value of calcite content can be obtained from the relative intensity Ic/(Ic + Iv), where Ic is the peak intensity of the calcite (104) plane (2θ = 29.3°) and Iv is that of the vaterite (101) plane (2θ = 27.0°).39 The calibration curve was obtained with variations of the ratio of calcite to vaterite content (see ESI†). The results of these measurements indicate that the calcite content levels of 1CaCO3 and 2CaCO3 were 59 and ∼100%, respectively. 2isoCaCO3 and 3CaCO3 were mainly composed of vaterite crystals (vaterite contents were 97 and 68%, respectively). Thus, polymer ligands 1 and 2 predominantly form calcite crystals, while 2iso and 3 produce vaterite crystals. | ||
| Fig. 6 XRD patterns of (a) 1CaCO3, (b) 2CaCO3, (c) 2isoCaCO3, (d) 3CaCO3, and (e) calcite crystals obtained in the absence of polymer. | ||
Fig. 7 shows the FE/SEM images of polymer ligand–CaCO3 composites. The FE/SEM image of CaCO3 crystals obtained in the absence of any polymer ligand is also depicted therein. It is known that a mixture of calcium chloride and ammonium carbonate without any polymer ligand results in the formation of the most stable polymorph, calcite, at room temperature.4 In the presence of our polymer ligands, 1CaCO3 and 2CaCO3 also yield calcite crystals (Fig. 7a and b). However, the crystal surface of these composites is different from calcite crystals in the absence of any ligand (Fig. 7d). The surfaces of 1CaCO3 and 2CaCO3 are craggy, although pure calcite crystals show a smooth surface. These differences are probably due to polymer binding to the CaCO3 crystal. On the other hand, 2isoCaCO3 and 3CaCO3 show vaterite crystals, which are rarely formed in nature. This finding also supports the polymer binding.
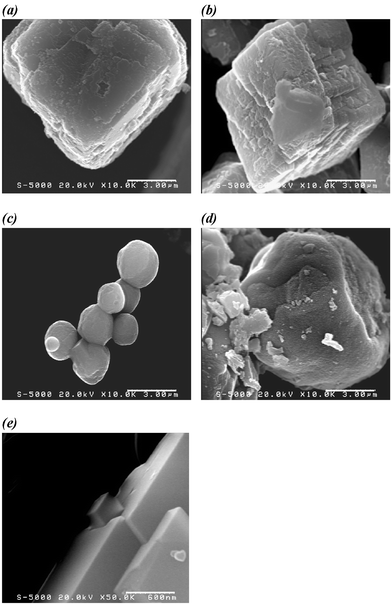 | ||
| Fig. 7 FE/SEM images of (a) 1CaCO3, (b) 2CaCO3, (c) 2isoCaCO3, (d) 3CaCO3, and (e) a calcite crystal in the absence of polymer (20 kV acceleration voltages). Scale bars in these images represent 3 µm for (a), (b), (c), and (d), and 600 nm for (e). | ||
Discussion
Effects of molecular weights of polymer ligands on CaCO3 crystallization
The number average molecular weights of polymer ligands, 1, 2, 2iso, and 3, are different. We think that the effects of molecular weights of polymers are negligible, because the effects of the NH⋯O hydrogen bonds are enough to affect the Ca-binding abilities of polymers on the Ca-binding site, and we also think some functional groups in biopolymers regulate the Ca-binding abilities in Ca-biominerals. The solution structures of high and low molecular weight polymer ligands are probably different from each other. However, if a polymer ligand forms a random coil, this polymer probably exhibits similar behavior to that of linear, zigzag chain, or helix coil polymers that are structure-controlled polymers. In this case, hydrogen-bonded units would exist at an effective position for Ca-binding. On the other hand, we suggest that it is enough for the Ca-binding that some hydrogen-bonded units exist in the Ca-binding site. Actually, only half of the carboxylate residues affect hydroxyapatite-binding in osteocalcin, which is a Ca-binding protein in bone.40 Therefore, the molecular weight of the polymer ligand does not need to be taken into consideration.Intra-side-chain NH⋯O hydrogen bonds in polymer ligands on crystallization of CaCO3 composites
The three types of polymer ligands, 1, 2iso, and 3, form the intra-side-chain NH⋯O hydrogen bonds in their carboxylate anion forms. The formation of the NH⋯O hydrogen bond stabilizes the Ca–O bond on the surface of the CaCO3 crystal and prevents its dissociation. These effects of the hydrogen bond on the Ca–O bond have been discussed in detail in our previous works.19,21 NH⋯O hydrogen bonds in carboxylic acid derivatives having neighboring amide NH groups lower the pKa value. Because of lowering the pKa value, the carboxylate anion is hardly protonated by water under neutral conditions on the surface of the CaCO3 composite. Actually, the intramolecular NH⋯O hydrogen-bonded benzoate derivative has a higher formation constant toward Ca2+ ion than the nonsubstituted benzoate without the NH⋯O hydrogen bond.21 Furthermore, our recent report indicates that the adhesion force toward calcium phosphate crystals with the intramolecular NH⋯O hydrogen-bonded polymer ligand, poly[1-carboxylate-2-(1-ethoxycarbonyl-3-methylsulfanylpropylcarbamoyl)-ethylene-alt-ethylene], is 5 times higher than the non-NH⋯O hydrogen-bonded poly(acrylate) ligand.41 Thus, the metal–oxygen bond is protected from hydrolysis by the intramolecular (or intra-side-chain) NH⋯O hydrogen bond.The polymer ligands hardly dislodge from CaCO3 crystals during the washing and still bind to CaCO3 crystals after the washing. On the other hand, when the CaCO3 is crystallized in the presence of three model ligands, 4, 5, and 6, the ligands are involved in the composites, and the model ligands are easily removed from CaCO3 crystals during the washing process (13C CP/MAS spectra and FE/SEM images were shown in the supplemental information). Our polymer ligands clearly bind to CaCO3 crystals. Thus, the intra-side-chain NH⋯O hydrogen bond in the polymer ligand plays an important role in the crystallization of CaCO3.
Effects of hydrogen bond strength toward CaCO3 polymorphs
The extract number of atoms which the intra-side-chain NH⋯O hydrogen bond ring comprises, affects to CaCO3 polymorphs. The alignment of the intra-side-chain in the polymer also influences the polymorphs. In the presence of syndiotactic polymers, the 5-membered ring hydrogen-bonded polymer 1 makes calcite crystals, whereas the 6-membered ring hydrogen-bonded polymer, 2iso, and the 7-membered one, 3, form vaterite crystals. These differences are due to the strength of the hydrogen bond. Polymer ligand 1 forms weak intra-side-chain NH⋯O hydrogen bonds just as its model ligand, 4; however, polymer ligands 2iso and 3 form strong hydrogen bonds similar to the models, 5 and 6, respectively. Formation of weak NH⋯O hydrogen bonds in a polymer ligand results in the relatively low stabilization of Ca–O bonds and restriction of CaCO3 crystal growth, and the obtained CaCO3 crystal is the most stable polymorph, calcite, without any restriction by the ligands. Actually, poly(acrylate) ligand without the hydrogen bond forms the most stable polymorph, calcite.5 In contrast, strong NH⋯O hydrogen-bonded polymer ligands stabilize one of the metastable polymorphs, vaterite, which is rarely found in nature.27,28 Thus, strong intra-side-chain NH⋯O hydrogen-bonded polymer ligands, such as 2iso and 3, restrict CaCO3 crystal growth to obtain vaterite crystals, although a weak intra-side-chain NH⋯O hydrogen-bonded ligand, such as 1, yields calcite crystals without any restricting crystal growth.Relationships between polymer tacticities and morphologies of CaCO3 composites
In the present study, the syndiotactic polymer ligand, 2, yields calcite crystals, although the isotactic-rich polymer ligand, 2iso, forms vaterite crystals. Polymer ligand 2iso forms strong intramolecular hydrogen bonds similar to its model, 5, because the degree of the downfield shift of the amide NH 1H NMR signal of 2iso from the COOH to the COO− forms is similar to that of 5 in Me2SO-d6. However, 2 does not exhibit a similar downfield shift. The hydrogen bond in 2 is weak and formed intermolecularly. Polymer ligand 2 clearly shows a different tendency from 2iso. Although 2 forms intermolecular NH⋯O hydrogen bonds in solution, some parts of the side-chains of 2 probably form intra-side-chain NH⋯O hydrogen bonds in the solid state, such as 2CaCO3. Actually, 2 kept the strong bonds to CaCO3 crystals after the washing process. This strong binding is probably due to the formation of the intra-side-chain NH⋯O hydrogen bonds.28,38 The hydrogen bond strength in 2 is weak, as in 1. Therefore, calcite crystals are obtained in the presence of polymer 2.In contrast, polymer 2iso hardly forms intermolecular hydrogen bonds because of the intra-side-chain static hindrance. Thus, 2iso strongly binds to CaCO3 crystal in the same way as was found in 3 by the strong formation of NH⋯O hydrogen bonds, and thus restricts the crystal growth to give vaterite crystals.
Conclusion
Novel poly(methacryloylaminocarboxylate) ligands were synthesized as model ligands of the acidic peptides in CaCO3 biominerals. These polymer ligands form 5-, 6-, or 7-membered-ring intra-side-chain or intermolecular NH⋯O hydrogen bonds between the carboxylate oxyanion and the neighboring amide NH. The strength of the NH⋯O hydrogen bonds in the polymer ligands restricts CaCO3 morphologies. The formation of weak hydrogen bonds in the polymer ligand does not affect the CaCO3 crystal growth; however, strong hydrogen bonds restrict crystal growth. Five-membered-ring intra-side-chain and intermolecularly NH⋯O hydrogen-bonded polymer ligand–CaCO3 composites form calcite crystals due to the formation of the weak hydrogen bond, while 6- or 7-membered-ring intra-side-chain NH⋯O hydrogen-bonded polymer ligand–CaCO3 composites form vaterite crystals because of the formation of the strong hydrogen bond. In conclusion, various CaCO3 morphologies can be regulated by slightly changing the strength of the hydrogen bonds in a polymer ligand.Acknowledgements
We acknowledge the support of this work by a Research Fellowship of the 21st Century Center Of Excellence Program “Integrated EcoChemistry” for Young Scientists [for K. Takahashi, 2002–2004] and a Grant-in-Aid for Scientific Research (A) (No. 12304040) from the Ministry of Education, Culture, Science, Sports, and Technology, Japan.References
- S. Mann, in Inorganic Materials—2nd Edition, ed. D. W. Bruce and D. O'Hare, John Wiley & Sons Ltd., Chichester, 1996, p. 255 Search PubMed.
- (a) S. Weiner, CRC Crit. Rev. Biochem., 1986, 20, 365 CrossRef CAS; (b) S. Mann, D. D. Archibald, J. M. Didymus, T. Douglas, B. R. Heywood, F. C. Meldrum and N. J. Reeves, Science, 1993, 261, 1286 CAS.
- (a) S. Mann, Struct. Bonding, 1983, 54, 125 CAS; (b) D. Jacquemain, S. G. Wolf, F. Leveiller, M. Deutsch, K. Kjaer, J. Als-Nielsen, M. Lahav and L. Leiserowitz, Angew. Chem., Int. Ed. Engl., 1992, 31, 130 CrossRef; (c) R. B. Frankel and S. Mann, Encyclopedia of Inorganic Chemistry, John Wiley & Sons Ltd., Chichester, 1994 Search PubMed; (d) A. M. Belcher, X. H. Wu, R. J. Christensen, P. K. Hansma, G. D. Stuky and D. E. Morse, Nature, 1996, 381, 56 CrossRef CAS; (e) J. Aizenberg, J. Hanson, T. F. Koetzle, S. Weiner and L. Addadi, J. Am. Chem. Soc., 1997, 119, 881 CrossRef CAS.
- L. Addadi and S. Weiner, Mol. Cryst. Liq. Cryst., 1986, 134, 305 CrossRef CAS.
- L. Addadi, J. Moradian, E. Shay, N. G. Maroudas and S. Weiner, Proc. Natl. Acad. Sci. USA, 1987, 84, 2732 CAS.
- L. Addadi and S. Weiner, Angew. Chem., Int. Ed. Engl., 1992, 31, 153 CrossRef.
- K. Takahashi, H. Yamamoto, A. Onoda, M. Doi, T. Inaba, M. Chiba, A. Kobayashi, T. Taguchi, T. Okamura and N. Ueyama, Chem. Commun., 2004, 996 RSC.
- S. Albeck, J. Aizenberg, L. Addadi and S. Weiner, J. Am. Chem. Soc., 1993, 115, 11691 CrossRef CAS.
- J. Aizenberg, G. Lambert, L. Addadi and S. Weiner, Adv. Mater., 1996, 8, 222 CAS.
- B.-A. Gotliv, L. Addadi and S. Weiner, ChemBioChem, 2003, 4, 522 CrossRef CAS.
- S. Raz, P. C. Hamilton, F. H. Wilt, S. Weiner and L. Addadi, Adv. Funct. Mater., 2003, 13, 480 CrossRef CAS.
- G. Falini, S. Albeck, S. Weiner and L. Addadi, Science, 1996, 271, 67 CrossRef.
- (a) S. Mann, J. M. Didymus, N. P. Sanderson, B. R. Heywood and E. J. A. Samper, J. Chem. Soc., Faraday Trans., 1990, 86, 1873 RSC; (b) T. Kato, T. Suzuki, T. Amamiya, T. Irie and M. Komiyama, Supramol. Sci., 1998, 5, 411 CrossRef CAS; (c) I. Lee, S. W. Han, H. J. Choi and K. Kim, Adv. Mater., 2001, 13, 1617 CrossRef CAS; (d) L. A. Estroff, C. D. Incarvito and A. D. Hamilton, J. Am. Chem. Soc., 2004, 126, 2 CrossRef CAS.
- G. Xu, N. Yao, I. A. Aksay and J. T. Groves, J. Am. Chem. Soc., 1998, 120, 11977 CrossRef CAS.
- K. Naka, Y. Tanaka, Y. Chujo and Y. Ito, Chem. Commun., 1999, 1931 RSC.
- J. J. J. M. Donners, R. J. M. Nolte and N. A. J. M. Sommerdijk, J. Am. Chem. Soc., 2002, 124, 9700 CrossRef CAS.
- D. Volkmer, M. Fricke, D. Vollhardt and S. Siegel, J. Chem. Soc., Dalton Trans., 2002, 4547 RSC.
- D. Rautaray, S. R. Sainkar and M. Sastry, Chem. Mater., 2003, 15, 2809 CrossRef CAS.
- (a) A. Onoda, Y. Yamada, M. Doi, T. Okamura and N. Ueyama, Inorg. Chem., 2001, 40, 516 CrossRef CAS; (b) A. Onoda, Y. Yamada, T. Okamura, M. Doi, H. Yamamoto and N. Ueyama, J. Am. Chem. Soc., 2002, 124, 1052 CrossRef CAS.
- A. Onoda, Y. Yamada, J. Takeda, Y. Nakayama, T. Okamura, M. Doi, H. Yamamoto and N. Ueyama, Bull. Chem. Soc. Jpn., 2004, 77, 321 CrossRef CAS.
- A. Onoda, Y. Yamada, Y. Nakayama, K. Takahashi, H. Adachi, T. Okamura, H. Yamamoto, N. Ueyama, D. Vyprachtiky and Y. Okamoto, Inorg. Chem., 2004, 43, 4447 CrossRef CAS.
- N. Ueyama, S. Moriyama, T. Ueno and A. Nakamura, in Peptide Science: Present and Future, Proceeding of the International Peptide Symposium, 1st, Kyoto, Nov. 30-Dec. 5, 1997, ed. S. Yasutsugu, Kluwer, Dordrecht, 1999, p. 288 Search PubMed.
- N. Ueyama, N. Nishikawa, Y. Yamada, T. Okamura and A. Nakamura, J. Am. Chem. Soc., 1996, 118, 12826 CrossRef CAS.
- (a) T. Ueno, Y. Kousumi, K. Yoshizawa-Kumagaya, K. Nakajima, N. Ueyama, T. Okamura and A. Nakamura, J. Am. Chem. Soc., 1998, 120, 12264 CrossRef CAS; (b) T. Okamura, S. Takamizawa, N. Ueyama and A. Nakamura, Inorg. Chem., 1998, 37, 18 CrossRef.
- (a) S. C. Tang, S. Koch, G. C. Papaefthymiou, S. Foner, R. B. Frankel, J. A. Ibers and R. H. Holm, J. Am. Chem. Soc., 1976, 98, 2414 CrossRef CAS; (b) W. R. Scheidt, D. K. Geiger, Y. J. Lee, C. A. Reed and G. Lang, J. Am. Chem. Soc., 1985, 107, 5693 CrossRef CAS.
- N. Ueyama, H. Kozuki, M. Doi, Y. Yamada, K. Takahashi, A. Onoda, T. Okamura and H. Yamamoto, Macromolecules, 2001, 34, 2607 CrossRef CAS.
- K. Takahashi, H. Kozuki, A. Onoda, T. Okamura, H. Yamamoto and N. Ueyama, J. Inorg. Organomet. Polym., 2002, 12, 99 CrossRef CAS.
- K. Takahashi, M. Doi, A. Kobayashi, T. Taguchi, A. Onoda, T. Okamura, H. Yamamoto and N. Ueyama, J. Cryst. Growth, 2004, 263, 552 CrossRef CAS.
- F. Sanda, T. Abe and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 2619 CAS.
- A. Gallardo and J. S. Román, Polymer, 1993, 34, 394 CrossRef CAS.
- (a) T. Nakano, M. Mori and Y. Okamoto, Macromolecules, 1993, 26, 867 CrossRef CAS; (b) A. Matsumoto, T. Matsumura and S. Aoki, Macromolecules, 1996, 29, 423 CrossRef CAS; (c) A. Matsumoto, K. Yokoi, S. Aoki, K. Tashiro, T. Kamae and M. Kobayashi, Macromolecules, 1998, 31, 2129 CrossRef CAS.
- (a) A. Dasgupta and S. Sivaram, Macromolecules, 1994, 27, 1665 CrossRef CAS; (b) S. Habaue, T. Uno, H. Baraki and Y. Okamoto, Macromolecules, 2000, 33, 820 CrossRef CAS; (c) C. L. Mero and N. A. Porter, J. Org. Chem., 2000, 65, 775 CrossRef CAS; (d) T. Kitayama, W. Shibuya and K.-i. Katsukawa, Polym. J., 2002, 34, 405 CAS.
- Y. Isobe, D. Fujioka, S. Habaue and Y. Okamoto, J. Am. Chem. Soc., 2001, 123, 7181.
- E. S. Stevens, N. Sugawara, G. M. Bonora and C. Toniolo, J. Am. Chem. Soc., 1980, 102, 7048 CrossRef CAS.
- T. Okamura, K. Sakaue, N. Ueyama and A. Nakamura, Inorg. Chem., 1998, 37, 6731 CrossRef CAS.
- M. Kato, T. Okamura, H. Yamamoto and N. Ueyama, J. Inorg. Biochem., 2003, 96, 165 CrossRef.
- N. Ueyama, T. Hosoi, Y. Yamada, M. Doi, T. Okamura and A. Nakamura, Macromolecules, 1998, 31, 7119 CrossRef CAS.
- K. Takahashi, M. Doi, A. Kobayashi, T. Taguchi, A. Onoda, T. Okamura, H. Yamamoto and N. Ueyama, Chem. Lett., 2004, 33, 192 CrossRef CAS.
- A. Katsifaras and N. Spanos, J. Cryst. Growth, 1999, 204, 183 CrossRef CAS.
- Q. Q. Hoang, F. Slcherl, A. J. Howard and D. S. C. Yang, Nature, 2003, 425, 977 CrossRef CAS.
- K. Takahashi, M. Doi, H. Mohri, T. Okamura, H. Yamamoto and N. Ueyama, Chem. Lett., 2004, 33, 1528 CrossRef CAS.
Footnote |
| † Electronic supplemental information (ESI) available: NMR signal assignments of polymer ligands, synthetic procedures of monomers for polymer ligands (MA-Val-OPac, MA-β-Ala-OBzl, and MA-γ-Abu-OtBu) and model ligands for the polymer repeated unit (4H, 5H, and 6H), 1H NMR spectra of 4H, 4NMe4, 5H, 5NMe4, 6H, and 6NMe4, 13C CP/MAS spectra of model ligand–CaCO3 composites, FE/SEM images of model ligand–CaCO3 composites, and the calibration curve of the ratio of calcite to vaterite content on an XRD. See http://www.rsc.org/suppdata/jm/b4/b415692g/ |
| This journal is © The Royal Society of Chemistry 2005 |

