Homeotropic alignment on surface-initiated liquid crystalline polymer brushes
Paul J.
Hamelinck
and
Wilhelm T. S.
Huck
*
Melville Laboratory for Polymer Synthesis, University of Cambridge, Cambridge, UK. E-mail: wtsh2@cam.ac.uk; Fax: +44 1223 334866; Tel: +44 1223 331509
First published on 19th November 2004
Abstract
With the development of new types of liquid crystal displays and the use of liquid crystals as organic semiconductors, homeotropic alignment layers have become more and more important. Liquid crystalline polymer (LCP) brushes have been suggested as an alignment layer and are expected to have more interaction with the liquid crystal phase than other homeotropic alignment layers. Here we report the synthesis of side chain liquid crystalline polymer brushes by surface-initiated Atom Transfer Radical Polymerisation (ATRP) of an acrylate functionalized mesogen on glass and silicon substrates. Brush thicknesses up to 20 nm were achieved. Substrates coated with a brush layer of this polymer were used for alignment of the nematic liquid crystal 5-cyanobisphenyl (5CB). Nearly defectless homeotropic alignment over several square centimetres is demonstrated. Microcontact printing has been used to obtain a patterned alignment layer.
Introduction
Alignment has been the key topic of research in the field of liquid crystal displays, but since LC materials are more and more used as semiconducting materials, alignment has become an important part of the research in organic electronics as well.1–4 Alignment of molecules on surfaces is one of the most important ways to improve device performance.5 For display applications a planar alignment of molecules is needed. Techniques to fabricate the alignment layers required include rubbing of a polymer layer or photoalignment of a layer containing azo dyes.6,7 More recent display technologies however, like reverse mode LCD, require homeotropic alignment (perpendicular to the surface) and tilted alignment.8 In electronic devices with a liquid crystalline active phase, homeotropic alignment has been used for fabricating FETs.9–11 Homeotropic alignment generally occurs when the anchoring energy is low,12 and the alignment layers are normally hydrophobic in nature, with the aliphatic tails of the LC molecules driven to the interface.Surface-initiated polymers are a class of polymers known to possess a high degree of order.13 In 1994 it was suggested in a theoretical study by Halperin and Williams that liquid-crystalline polymer (LCP) brushes could be used as alignment layers.14 Polymer brushes were expected to have more interaction with the liquid crystal phase than other alignment layers, and the alignment is assumed to be dependent on grafting density. Dense main chain LC polymers would result in homeotropic alignment because the polymer chains are more stretched. When the grafting density is low, planar alignment is expected. These polymers would thus provide a means of tuning both the pre-tilt angle and the surface anchoring energy via grafting density. Monte Carlo simulations were consistent with this theory and show that these predictions also hold for short chains of four monomers.15
Peng et al. synthesised and studied side chain liquid crystalline surface-initiated polymers by free radical polymerization aiming to use them as alignment layers.16,17 Because of the orientation of side chains with respect to the main chain, the brushes were expected to induce planar alignment, so that the combination of brushes and treated substrate would allow tuning of the pre-tilt angle. Growing polymers from untreated surfaces however did not result in a preferred alignment.16 Polymers grown from rubbed surfaces resulted in an anisotropy in the 230 nm thick brushes observable by polarising microscopy.17 Liquid crystalline material showed planar alignment in the rubbing direction when injected into a capillary cell of these substrates, but the alignment would have been the same on pretreated substrates without a brush layer. Here we present the synthesis of side chain liquid crystalline polymer brushes grown from untreated substrates and their application as a homeotropic alignment layer.
Experimental
General procedures
Ellipsometric measurements were carried out using a EL X-02C ellipsometer from Dr Riss Ellipsometerbau GmbH with a 632.8 nm laser at 70° angle of incidence. Refractive indices of 1.50 and 1.45 were used for polymer and initiator layers respectively.FT-IR spectra were taken using a Bio-Rad FTS 6000 spectrometer. Spectra of surface-initiated polymers and spin coated monomers were taken in transmission mode using a background of the same initiator coated wafer that was used for polymer growth. Plasma oxidation of substrates was performed in air in an Emitech K1050X plasma oxidiser for 10 min at 100 W.
Polarised light microscopy was performed on a Nikon Eclipse ME600 microscope with a Nikon DN100 digital net camera connected to it.
Materials
Reactive mesogen RM488 was donated by Merck Chemicals Ltd, Chilworth, Southampton. Dow Corning Sylgard 184 Base silicon elastomer and Sylgard 184 Silicon curing agent were purchased from VWR. All other chemicals were purchased from Aldrich, Lancaster or Fisher and used as received unless otherwise indicated. Triethylamine was distilled from and stored over potassium hydroxide. Toluene was distilled from sodium and stored over molecular sieves. Copper(I) chloride and copper(I) bromide were 99+% and 99.999% purity respectively and were stored in a glove box. Dichloromethane, ethyl acetate, acetone, toluene and hexane were distilled prior to use. Methanol and ethanol were Analytical Reagent grade and used as received. The trichlorosilane initiator (2-bromo-2-methylpropionic acid 3-trichlorosilanylpropyl ester) was synthesised following a reported procedure,18 however using allyl alcohol instead of 5-hexene-1-ol. PDMS Stamps were fabricated following reported procedures.19 Silicon wafers were obtained from Compart Technology Ltd. (100 mm diameter, phosphorus-doped, <100> orientation, polished one side).Immobilisation of the initiator monolayer on the substrate
Silicon wafers were plasma oxidised before functionalization. Glass samples were sonicated for 2 minutes in a soap solution, subsequently for 2 minutes in demineralised water and finally for 2 minutes in ethanol and dried in a nitrogen stream. After this physical cleaning step they were oxidised in the plasma oxidiser.For initiation of the entire surface, the silicon and/or glass substrates were placed in a crystallising dish and 30 mL of dry toluene, 50 µL of triethylamine and 10 µL of the trichlorosilane initiator were added. The dish was covered and left overnight at room temperature. The wafer was then washed sequentially with toluene, distilled acetone and absolute ethanol and dried under a nitrogen stream.
For a patterned immobilisation of the initiator monolayer on glass substrates by microcontact printing (μCP), a flat piece of PDMS was used as an “ink pad”. This piece was soaked in a solution of 5 µL of the trichlorosilane initiator in 20 mL of hexane and blown dry in a nitrogen stream for 60 s. A patterned PDMS stamp was inked by putting it on the flat piece of PDMS and leaving it for 30 s without applying additional pressure. The stamp was then transferred to the glass substrate and left there for 30 s without applying additional pressure. The substrate with the pattern of the trichlorosilane initiator was then rinsed with subsequently hexane, dichloromethane and ethanol and dried in a stream of nitrogen.
Synthesis of polymer brushes of RM 488 on silicon (typical procedure)
The reaction mixture and stock solutions were prepared in the glove box. 4 g (7.83 mmol) of RM488 was dissolved in 4 mL of degassed DMF and 2 mL of degassed acetone at 50 °C and transferred to a petri dish containing initiated silicon and or glass samples. 80 µL of a solution of 96 mM copper(I) chloride (0.80 mg, 7.7 µmol) and 29 mM copper(II) bromide (0.52 g, 2.3 µmol) in DMF, 0.8 mL of a solution of a 6.7 mM solution of copper(II) chloride (0.53 mg, 5.4 µmol) in DMF and 120 µL of a 0.32 M 2,2′-bipyridyl (BPY) solution (6.1 mg, 38 µmol) in DMF were added to the reaction mixture. The petri dish was covered and sealed with parafilm to avoid evaporation of the solvents. Surface-initiated polymerization was performed at 50 °C in the glove box for 24 h. After the reaction, the substrates were subsequently washed in dichloromethane for 2 minutes under sonication, in toluene, in water and in ethanol and then dried in a stream of nitrogen. The substrates were then stored under nitrogen until further characterisation and use. Ellipsometric thickness: 20 nm. IR: νmax/cm−1: 2958 (alkyl C–H stretch, s), 2108 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretch, w), 1735 (C
C stretch, w), 1735 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, s), 1605 (phenyl, m), 1502 (phenyl, m).
O stretch, s), 1605 (phenyl, m), 1502 (phenyl, m).
Fabrication of capillary LC cells
Two thin plastic spacers (thickness ∼ 10 µm, cling film) were placed 3 mm from the edges of an upward facing substrate. A second substrate was glued on top of the first substrate, using two drops of cyanoacrylate glue between the edges and the spacers. Both the nematic liquid crystal 4′-pentyl-4-biphenylcarbonitrile (5CB) and the capillary cell were heated to 40 °C on a heating plate. At this temperature 5CB is an isotropic liquid. 5CB was then injected into the capillarity of the cell. The cell was cooled down to room temperature before further characterisation.Results and discussion
Synthesis of side chain liquid crystalline polymer brushes
Surface-initiated polymers were grown with reactive mesogen RM488 on glass and silicon, which resulted in polymer brushes with liquid crystalline side chains (Scheme 1). Different reaction conditions were investigated by varying the copper(I) halide and copper(II) halide concentrations, with bromide and chloride used as halides, and by varying the temperature and solvents. The reaction conditions used eventually were 50 °C, copper(I) chloride as catalyst, 2,2′-bipyridyl being used as the ligand, copper(II) bromide and copper(II) chloride being added for more living character with a 1 : 0.7 : 0.3 : 5 : 1000 CuCl : CuCl2 : CuBr2 : BPY : RM488 mole ratio. By changing the halide from bromide to chloride the living character was enhanced as the C–Cl bond is stronger than the C–Br bond, thus favouring the dormant state. A mixed halide system, in which both chloride and bromide are present, was used to balance the advantages of fast brush growth with enhanced living character.20 The solvent mixture used was a 2 : 1 (v/v) DMF–acetone mixture at a concentration of 1 g monomer per 1.5 mL of this mixture. Although the solubility in toluene was higher than in the DMF–acetone mixture, the use of this mixture resulted in thicker brushes. Faster brush-growth in solvents with higher dielectric constants is common for ATRP.21 The time resolved growth of surface-initiated polymers grown from silicon is shown in Fig. 1 for different reaction conditions and the graph clearly shows the enhancement of the living character upon adding copper(II) and upon changing to a mixed halide system. Brush thicknesses of up to 20 nm were achieved in 24 h following the procedure mentioned above. The film remains stable during intensive cleaning steps including two minutes rinsing in dichloromethane and toluene in an ultrasonic bath. | ||
| Fig. 1 Surface-initiated growth of polymer brushes of RM488 on silicon. The lines are given as guides to the eye. | ||
 | ||
| Scheme 1 | ||
At temperatures higher than 60 °C polymer formation in the reaction solution was also observed, independent of solvent used; either toluene or DMF. This is either a result of radical transfer from chains growing on the surface, or, more likely, from auto-initiation in the polymerization solution, as it did not occur at room temperature.
Alignment of liquid crystals on surface-initiated polymers
Glass substrates with surface-initiated polymers were used to construct LC cells. Two substrates were glued on top of each other with a spacer (∼10 µm) in between (Fig. 2). The nematic LC 5CB was injected in the resulting capillary space and alignment was studied with polarised light microscopy having the two polarizers crossed.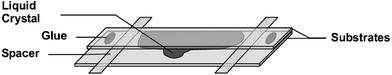 | ||
| Fig. 2 Schematic structure of a capillary LC cell. The liquid crystal is injected in the space between the two substrates and spreads as a result of capillary forces. | ||
Samples with 5 nm and 8 nm thick brushes showed remarkably homogeneous homeotropic alignment over the entire area of the cells (several cm2, Fig. 3). Homeotropic alignment in which the axis of the LC molecules is perpendicular to the substrate was identified in several different ways. The extinction when studied with crossed polarizers did not change upon rotation of the sample in the plane of the viewing stage although the sample was not in the isotropic phase. This is because the axis of the homeotropically aligned LC molecules is perpendicular to the viewing direction. Apart from that, the characteristic interference pattern—a Maltese cross—was observed under illumination with convergent light. Alignment was not observed in LC cells of plain glass or in LC cells of initiator-coated glass substrates without a brush layer or with a PMMA brush layer.
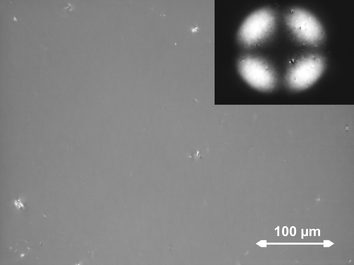 | ||
| Fig. 3 Homeotropic alignment over large areas in LC cells of substrates with 5 nm thick brushes of RM488 observed between crossed polarizers with normal illumination. The inset shows the interference pattern observed with convergent illumination. | ||
To study the influence of brush thickness on the alignment, we also fabricated LC cells with 20 nm thick brush samples (not shown). In these samples there were still large areas of homeotropic alignment, but interspersed with areas where the extinction changed upon rotation of the cells in the plane of the viewing stage. The observed interference pattern was a tilted cross. This indicates that the alignment is somewhat between planar and homeotropic, i.e. tilted. No phase boundaries were visible between the areas of homeotropic and tilted alignment. As shown in Fig. 1, at the reaction time for 20 nm thick brushes, the reaction rate is no longer in the linear regime, which indicates the polymerization is not controlled and many chains have terminated. This results in higher conformational freedom of the chains at the surface which could explain the decreased alignment of the nematic phase.
As expected from the study of Halperin and Williams and the experimental work by the group of Rühe,16,17 the alignment on side chain liquid crystalline polymer brushes should be planar. However in this study we observed homeotropic alignment on brushes. This difference can be explained by either rejecting the assumption that the bulky side chains have an orientation perpendicular to the main chain or by assuming that the brush backbones are not perfectly perpendicular to the substrate. The side chains could have another orientation because of steric interactions or because of solvation effects, when the liquid crystal molecules penetrate the brush layer. The polymer backbones in brushes are not totally stretched out but still have a certain amount of coiling dependent on the grafting density.22,23 This coiling could be responsible for a homeotropic orientation of the side chains, thus inducing homeotropic alignment in the liquid crystal layer. We have investigated the effect of grafting density of the brushes on liquid crystal alignment. However, initial results indicate that even diluting the brushes by a factor of ten (using a mixed monolayer of initiator and propyl trichlorosilane in a 1 : 9 ratio) did not lead to an appreciable decrease in alignment ‘power’. This does indeed suggest that disordered brushes with homeotropically aligned side chains are responsible for the observed alignment.
Rühe and co-workers did not observe homeotropic alignment.16,17 There are however two differences between their experiments and ours. Firstly, they grew much thicker brushes of over 200 nm. Secondly, they use a different polymerisation technique, namely free radical polymerisation. The latter results in higher polydispersities compared to the controlled radical polymerisation we use. Higher polydispersities and longer polymer chains result in a higher conformational freedom of the chains and their side groups, especially at the brush/nematic interface. This could explain why homeotropic alignment is not observed in their experiments.
We feel that alignment on surface-initiated polymers is intrinsically different from alignment on self assembled monolayers. Reznikov et al. found in experiments on quartz substrates that an adsorbed monolayer of 5CB is oriented perpendicular to the substrate, but that the bulk alignment in the cell is planar with respect to the substrate.24 This alignment is explained by the alignment of dimers of 5CB (without net dipole) on the hydrophobic chains at the top of the absorbed layer. Apparently more liquid crystalline interactions between the liquid crystals at the surface and the bulk liquid crystals are needed to result in a homeotropic alignment. In the case of brush layers already a small polydispersity can provide these interactions.
Patterned alignment
Microcontact printing was used to deposit a patterned self assembled monolayer of the initiator before growing brushes. Brushes were grown from these substrates to study whether patterned alignment could be obtained. Fig. 4 shows the alignment of the liquid crystalline phase on these patterned brushes for a pattern of 200 µm wide hexagons and for 5 µm wide lines (in a periodicity of 15 µm). For the fabrication of these LC cells we used one substrate with patterned brushes and one plain glass substrate to be able to study the alignment on one of the substrates only. It can clearly be seen that the liquid crystal aligns only on the brushes and not on the background regions. When two substrates with different patterns are used to form an LC cell, the observed pattern shows features of both patterns (Fig. 5). The homeotropic alignment is strongest in those regions where brushes are present on both the top and bottom substrate.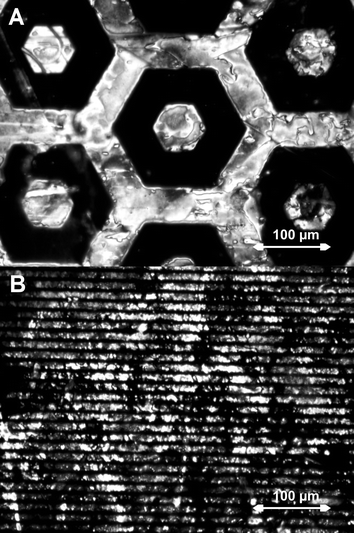 | ||
| Fig. 4 Patterned alignment on brushes grown from initiator immobilised by μCP. A) 200 µm wide hexagons; B) 5 µm wide lines in a 15 µm periodicity. The dark areas have homeotropic alignment; the light areas have no preferential orientation. | ||
 | ||
| Fig. 5 Patterned alignment in LC cells with a hexagon patterned top substrate and a line patterned bottom substrate. The homeotropic alignment shows features of both patterns and is strongest where brushes are present on both substrates. | ||
Conclusions
In this study we showed strong homeotropic alignment over large areas on surface-initiated polymers with liquid crystalline side chains. The alignment is the result of interactions between the liquid crystalline side chains of the polymer and the molecules in the liquid crystal phase and does not occur on substrates without a polymer layer or with polymer brushes of PMMA. Alignment occurs on substrates that reacted for only 30 minutes with brushes of only 5 nm thick and is more homeotropic on thin brushes than on thicker brushes. When the thickness is increased, the alignment changes to a more tilted alignment. Printing down a pattern of the initiator before brush growth results in a patterned alignment of the LC material.The homeotropic alignment over large areas and the ability to obtain patterned alignment by depositing the initiator with soft lithographic techniques make this procedure a promising tool for display and organic electronic applications.
Acknowledgements
We thank Merck Chemicals Ltd. for the donation of reactive mesogen RM488. We also thank Merck, the Cambridge European Trust, the Isaac Newton Trust and the IRC in Nanotechnology for financial support.References
- L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, E. Moons, R. H. Friend and J. D. Mackenzie, Science, 2001, 293, 1119 CrossRef CAS.
- N. Yoshimoto and J.-I. Hanna, J. Mater. Chem., 2003, 13, 1004 RSC.
- M. Grell and D. D. C. Bradley, Adv. Mater., 1999, 11, 895 CrossRef CAS.
- C. D. Simpson, J. Wu, M. D. Watson and K. Müllen, J. Mater. Chem., 2004, 14, 494 RSC.
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, M. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herig and D. M. de Leeuw, Nature, 1999, 401, 685 CrossRef CAS.
- M. O'Neill and S. M. Kelly, J. Phys. D: Appl. Phys., 2000, 33, R67 CrossRef CAS.
- L. T. Thieghi, J. J. Bonvent, E. A. Oliveira, J. A. Giacometti and D. T. Balogh, Appl. Phys. A, 2003, 77, 911 CrossRef CAS.
- Y. Suzuki, N. Mizoshita, K. Hanabusa and T. Kato, J. Mater. Chem., 2003, 13, 2870 RSC.
- A. M. van de Craats, N. Stutzmann, O. Bunk, M. M. Nielsen, M. Watson, K. Müllen, H. D. Chanzy, H. Sirringhaus and R. H. Friend, Adv. Mater., 2003, 15, 495 CrossRef CAS.
- I. McCulloch, W. Zhang, M. Heeney, C. Bailey, M. Giles, D. Graham, M. Shkunov, D. Sparrowe and S. Tierney, J. Mater. Chem., 2003, 13, 2436 RSC.
- H. Sirringhaus, F. J. Wilson, R. H. Friend, M. Inbasekaran, W. Wu, E. P. Woo, M. Grell and D. D. C. Bradley, Appl. Phys. Lett., 2000, 77, 406 CrossRef CAS.
- B. Alkhairalla, N. Boden, E. Cheadle, S. D. Evans, J. R. Henderson, H. Fukushima, S. Miyashita, H. Schönherr, G. J. Vancso, R. Colorado, jr., M. Graupe, O. E. Shmakova and T. R. Lee, Europhys. Lett., 2002, 59, 410 CrossRef CAS.
- S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14 RSC.
- A. Halperin and D. R. M. Williams, J. Phys. Condens. Matter, 1994, 6, A297 CrossRef.
- H. Lange and F. Schmid, J. Chem. Phys., 2002, 117, 362 CrossRef CAS.
- B. Peng, D. Johannsmann and J. Rühe, Macromolecules, 1999, 32, 6759 CrossRef CAS.
- B. Peng, J. Rühe and D. Johannsmann, Adv. Mater., 2000, 12, 821 CrossRef CAS.
- M. Husseman, E. E. Malmström, M. McNamara, M. Mate, D. Mecerreyes, D. G. Benoit, J. L. Hedrick, P. Mansky, E. Huang, T. P. Russell and C. J. Hawker, Macromolecules, 1999, 32, 1424 CrossRef.
- Y. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550 CrossRef CAS.
- W. Huang, J.-B. Kim, M. L. Bruening and G. L. Baker, Macromolecules, 2002, 35, 1175 CrossRef CAS.
- D. M. Jones and W. T. S. Huck, Adv. Mater., 2001, 13, 1256 CrossRef CAS.
- T. Wu, K. Efimenko, P. Vlček, V. Šubr and J. Genzer, Macromolecules, 2003, 36, 2448 CrossRef CAS.
- D. M. Jones, A. A. Brown and W. T. S. Huck, Langmuir, 2002, 18, 1265 CrossRef CAS.
- Y. Reznikov, O. Ostroverkhova, K. D. Singer, J.-H. Kim, S. Kumar, O. Lavrentovich, B. Wang and J. L. West, Phys. Rev. Lett., 2000, 84, 1930 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
