A continuous-flow dialysis system with inductively coupled plasma optical emission spectrometry for in vitro estimation of bioavailability†
Kunchit
Judprasong
ab,
Mathuros
Ornthai
a,
Atitaya
Siripinyanond
a and
Juwadee
Shiowatana
*a
aDepartment of Chemistry, Faculty of Science, Mahidol University, Rama VI Rd., Bangkok 10400, Thailand. E-mail: scysw@mahidol.ac.th; Fax: 66 2 3547151; Tel: 66 2 2015122
bOn study leave from Institute of Nutrition, Mahidol University, Salaya, Putthamonthon, Nakorn Pathom 73170, Thailand. E-mail: nukjp@mahidol.ac.th; Fax: 66 2 4419344; Tel: 66 2 8002380
First published on 22nd September 2005
Abstract
A continuous-flow dialysis (CFD) method with on-line inductively coupled plasma optical emission spectrometric (ICP-OES) simultaneous multielement measurement for the study of in vitro mineral bioavailability was developed. The method was based on a simulated gastric digestion in a batch system followed by a continuous-flow intestinal digestion. The simulated intestinal digestion was performed in a dialysis bag placed inside a channel in a flowing stream of dialyzing solution (NaHCO3). The mineral concentrations in the dialysate were determined by ICP-OES using Y and Sc as internal standards. The pH of the dialysate was also monitored on-line to ensure pH changes similar to the situation in the gastrointestinal tract.The developed system was applied to determining the dialysability of five essential elements (Ca, Mg, P, Fe, Zn) for various kinds of foods, i.e., milk-based infant formula reference material (NIST SRM 1846), milk powder, kale, mungbean, chicken meat, jasmine rice, and Acacia pennata. The dialysis profiles of elements and pH change profiles can be useful in understanding the dialysis change and factors affecting dialysability. All studied elements were rapidly dialysed in the first 30 min of simulated intestinal digestion. It is expected that this system will be useful for estimation of dialysability and for studying the mutual effects of components in food.
Introduction
Mineral deficiency is usually caused by a low mineral content in the diet when rapid body growth is occurring and/or when minerals from the diet are poorly absorbed. Mineral bioavailability has usually been determined by in vivo measurement.1 Ideally, mineral bioavailability studies should be performed in vivo and in humans; however, they are difficult, expensive, and provide limited data with each experiment.2 While animal assays are less expensive, they are somewhat limited by uncertainties with regard to differences in metabolism between animals and humans. As an alternative to human and animal in vivo studies, the availability of minerals or trace elements has also been estimated by simple, rapid and inexpensive in vitro methods.3 The earlier in vitro methods estimated bioavailability by measuring the dialysability of minerals through a semi-permeable membrane in equilibrium after simulated enzymatic digestion of foods, which was known as “Miller’s method”. However, in this equilibrium method, the dialysed components are not continuously removed, as occurrs in the intraluminal digestive tract. Many modifications have been made to Miller’s method in an attempt to improve the analytical methodology. Continuous-flow dialysis (CFD) in vitro methods were developed4–6 in which dialysed components were continuously removed. These methods measure the fraction of the available mineral pool in diets which is potentially capable of absorption. Although a true absorption is not determined, in vitro methods have frequently been used to predict and compare the availability of different foods because they are simple, rapid, inexpensive and easy-to-control. In addition, certain parameters can be monitored during in vitro dialysis. Some studies reported poor correlation between in vitro and in vivo bioavailability,7,8 whereas some studies reported good correlation between results obtained from the two methods.9,10 An example of disagreement between the two methods was reported for bioavailability estimation of Ca.8 The in vivo studies of Ca showed higher values than in vitro studies because some of the Ca bound species was released into the large intestine and might be absorbable.8 Therefore, to obtain a reliable and meaningful in vitro bioavailability study, a clear need exists for the development of a dialysis method that can mimic in vivo functions.A CFD system, by which dialysed components were continuously removed during simulated intestinal digestion, has been developed to obtain a closer simulation of the mineral absorption in the body.6 It involves an in vitro gastric digestion in a batch system (mimicking digestion in the stomach where no mineral absorption takes place), then intestinal digestion in a CFD system. The CFD in the intestinal digestion step enables dialysable components to be continuously removed for element detection. Moreover, the proposed CFD system offers information on dialysis kinetics, which could be extrapolated to be of some use for absorption studies. In our previous reports, the CFD system was operated with off-line flame AAS,6 and on-line electrothermal AAS detection11 for the study of Ca and Fe dialysability, respectively. However, many essential minerals are of nutritional interest, for example Ca and P are two essential elements for optimal bone mineralization.12 The roles of other major and trace elements (Mg, Fe, Zn, Cu, Se, etc.) are also of particular interest. Magnesium, Zn, and Fe serve metabolic and enzymatic functions. Zinc is essential for normal growth and development of the immune response.13 So, the CFD system with multielement detection capability for the determination of major and trace elements was aimed in this direction. An inductively coupled plasma optical emission spectrometric (ICP-OES) detection was chosen for this study. An ICP-OES spectrometer was connected on-line sequentially after an on-line pH measurement module to the CFD system to continuously monitor the dialysed multielement content and pH change during dialysis. The developed method was validated and applied for determination of mineral (Ca, Mg, P, Zn and Fe) dialysability for various kinds of food.
Experimental
Reagents and solutions
All reagents were of analytical grade, and ultrapure water of 18 MΩ cm specific resistivity obtained from a Milli-Q purification system (Millipore Corp., MA, USA) was used throughout. Glass and polyethylene containers were soaked in 10% nitric acid for at least 24 h and then rinsed three times with ultrapure water before use.A pepsin solution was prepared by dissolving 0.16 g of pepsin (P-7000, porcine stomach mucosa, Sigma, St.Louis, MO, USA) in 1 mL of 0.1 mol L−1 HCl. A pancreatin–bile extract (PBE) mixture was prepared by dissolving 0.004 g of pancreatin (P-1750, porcine pancreas, Sigma) and 0.025 g of bile extract (B-6831, porcine, Sigma) in 5 mL of 0.001 mol L−1 NaHCO3. Flat dialysis membranes (MWCO 12–14 kDa) 10 mm wide and 17.6 cm in length (cellu-Sep®H1, Membrane Filtration Products, Texas, USA) were used in the intestinal digestion procedure. The membranes were boiled for 30 min in 40% ethanol, soaked in 1 mM ethylenediamine tetraacetic acid (EDTA; BDH Ltd., Poole, UK) for 30 min, rinsed several times with Milli-Q water, stored in 0.01 M NaHCO3 and rinsed with Milli-Q water before use. A multielemental stock solution (QCS 01–5 at 100 μg mL−1), Y (ICP-69N-1 at 1000 g mL−1) and Sc (ICP-53N-1 at 1000 g mL−1), as internal standards, were from AccutraceTM (AccuStandard®, USA). Standard solutions were prepared immediately before use by dilution of stock standard with 2% HNO3.
Instrument and equipment
The CFD system used in this study was described elsewhere.6 The outlet of the CFD system was connected to a pH measurement module and ICP-OES detection unit (Fig. 1). A shaking water bath (Memmert®, Memmert GmbH, Germany), controlled at 37 ± 1 °C, was used for both gastric and intestinal digestions. The Orion SensorLink pH measurement system (ThermoOrion, USA), Model PCM500, equipped with PCMCIA slot and personal computer, was used to determine pH during digestion and dialysis. | ||
| Fig. 1 Schematic diagram of continuous-flow dialysis system with on-line ICP-OES and pH measurements. A 2.5 mL pepsin digest sample was injected into the dialysis bag using a syringe. A 625 μL aliquot of pancreatin–bile extract was introduced into the dialysis bag after the first 30 min of the dialysis process. Dialysis was continued for 150 min. The dialysate flow was sequentially subjected to pH and ICP-OES measurements. | ||
Determination of minerals by ICP-OES was performed using a SPECTRO CIROS CCD, axial configuration, equipped with a glass spray chamber (double pass, Scott-type) and a cross-flow nebulizer (all from SPECTRO Analytical Instruments, Germany). The ICP-OES operating conditions were as follows: power 1350 W; nebulizer gas flow 1 L min−1; and auxiliary gas flow 12 L min−1. Selected emission lines were: Ca, 396.847 (II), 317.933 (II), and 422.673 (I); Mg, 279.553 (II), 279.079 (II) and 280.270 (II); P, 177.495 (I) and 214.914 (I); Fe, 238.204 (II), 239.562 (II), 259.940 (II); and Zn, 202.548 (II), 206.191 (II), 213.856 (II) nm. Emission lines for internal standards were: Y, 320.332 (II), 371.030 (II) and 442.259 (II); and Sc: 256.023 (II), 361.384 (II), and 440.037 (II) nm. A closed microwave digestion unit (Milestone MLS 1200 mega, Italy) equipped with 6 Teflon vessels was used to mineralize 0.5–1 g of food samples with 10.0 mL of concentrated nitric acid prior to determination of the total mineral contents by ICP-OES.
Sample collection and preparation
Milk-based infant formula (NIST SRM 1846) was used for method validation. Representative samples of cow milk, kale (Brassica oleracea var. alboglabra, Bail.), Acacia pennata (L., Willd. Subsp.), mungbean (Phaseolus aureus Roxb.), chicken meat, and jasmine rice (Orya sativa) were examined. Three samples for each food item, 1–3 kg, were purchased randomly in three local markets in metropolitan areas of Bangkok, Thailand. After purchase, the samples were transported as soon as possible to the laboratory. Milk powder, jasmine rice, and mungbean were used as purchased. Kale, Acacia pennata and chicken meat were cleaned once with tap water, and twice with Milli-Q water. The inedible portions of each sample were recorded and discarded. The edible parts in all samples except milk powder and infant formula were cooked by boiling, homogenized by a food processor (Tefal® Kaleo Blender, France), kept in an acid-washed screw-capped plastic bottle, and stored at −20 °C. A representative portion of the homogenized samples was dried at 60 °C and ground to fine particles, and then stored in a sealed plastic bag in a desiccator at room temperature until analysis.Analytical procedure
For intestinal digestion and dialysis, a dynamic CFD system was used (Fig. 1). A portion of the mixture after gastric digestion (2.0–2.5 g) was injected into the flattened dialysis bag in the dialysis chamber via a syringe. The dialysing solution, NaHCO3 of optimum concentration, determined by titratable acidity6 for each food sample as summarized in Table 1, flowed through the outer surface of the bag at 1 mL min−1 and the temperature was controlled at 37 °C. The dialysable components in the dialysing solution were transported into the pH measurement cell and finally to the ICP-OES. To obtain good nebulization performance and to ensure that the analyte elements remained soluble, the stream of dialysing solution was acidified by mixing with a stream of 4% nitric acid, which also contained 1 mg L−1 of Y and Sc, used as internal standards at 1.0 mL min−1 (see Fig. 1). Blanks for gastric and intestinal digestions were also performed in each experiment to control possible contamination problems.
| Sample | Optimum NaHCO3/mol L−1 |
|---|---|
| Jasmine rice | 7.77 × 10−4 |
| Chicken meat | 1.89 × 10−3 |
| Mungbean | 1.32 × 10−4 |
| Acacia pennata | 1.59 × 10−3 |
| Milk powder | 1.14 × 10−3 |
| Kale | 1.44 × 10−3 |
In the CFD method, the concentration of each mineral in the dialysate (μg mL−1) was obtained by on-line ICP-OES measurement by external calibration using freshly prepared standard solution in NaHCO3 of similar concentration to the dialysing solution, which was acidified to contain 2% HNO3 before use. The total amount of dialysed minerals was determined by integration of the signal through the whole dialysis time using a computer program (Microcal Origin, Version 6.0).
Dialysability in percent was calculated as follows: dialysability (%) = 100 × D/C, where D represents dialysed mineral content (μg g−1 sample) and C represents the total mineral content (μg g−1 sample). After dialysis, mineral contents in the residue inside the dialysis bag were determined by ICP-OES after acid digestion, and the percentages of mineral remaining were calculated.
Results and discussion
I. Setup of continuous-flow dialysis system with on-line detections
The design of the continuous-flow dialysis (CFD) system for in vitro determination of mineral bioavailability, the selection of dialysing solution flow rate and optimization of dialysing solution (NaHCO3) have been reported elsewhere.6 The optimum flow rate of dialysing solution in the CFD and the uptake rate of ICP-OES were similar at 1 mL min−1, therefore they can be readily coupled. Nonetheless, to obtain good nebulization performance, sample solution was acidified before reaching the nebulizer by merging the stream of sample solution with a stream of 4% nitric acid. The signal intensities of Ca and Fe in acidified samples were increased remarkably, in comparison with those which were not acidified.II. Validation of analytical method
Because a reference material, which would provide bioavailability data, is not available, validation of the proposed method was performed by studying the analytical recoveries of all elements of interest. Milk-based infant formula reference material (SRM 1846) was subjected to the proposed analytical procedure to determine the dialysable minerals in the dialysate and the non-dialysable ones in the retentate. The non-dialysable mineral in the retentate was determined by ICP-OES after acid digestion of the remaining of food suspension in the dialysis tube. The total mineral content of the sample was determined after total digestion of the sample. The results are given in Table 2. The results of total concentration obtained by ICP-OES after acid digestion agreed well with the certified values for all elements studied, suggesting good performance of ICP-OES detection. In addition, a summation of the dialysed minerals (by CFD-ICP-OES) and non-dialysed minerals contents was similar to the certified values with recoveries ranging from 90 ± 5% for Fe to 104 ± 6% for P, suggesting good performance of the CFD system and the absence of matrix interferences from the dialysable components and therefore the reliability of an on-line ICP-OES determination of dialysable minerals without prior acid digestion. With the proposed CFD-ICP-OES system, a quantitative recovery was obtained.| Mineral contents | |||||
|---|---|---|---|---|---|
| Ca | Mg | P | Fe | Zn | |
| a Blank subtracted. | |||||
| Certified value/μg g−1 | 3670 ± 200 | 538 ± 29 | 2610 ± 150 | 63.1 ± 4.0 | 60.0 ± 3.2 |
| Total minerals/μg g−1a | 3760 ± 160 | 541 ± 17 | 2490 ± 96 | 66.6 ± 1.5 | 62.8 ± 1.8 |
| dialysed minerals/μg g−1a | 2800 ± 140 | 428 ± 10 | 1730 ± 200 | 17.7 ± 2.2 | 51.5 ± 3.7 |
| Non-dialysed minerals/μg g−1a | 792 ± 46 | 86 ± 12 | 1040 ± 94 | 42.4 ± 5.5 | 7.8 ± 1.0 |
| dialysed + non-dialysed mineral/μg g−1 | 3600 ± 140 | 514 ± 6 | 2690 ± 145 | 60.1 ± 5.0 | 59.0 ± 4.9 |
| Dialysis (%) | 76 ± 4 | 80 ± 2 | 70 ± 5 | 27 ± 3 | 82 ± 6 |
| Element retained (%) | 22 ± 1 | 16 ± 2 | 42 ± 4 | 64 ± 5 | 18 ± 2 |
| Sum (%) | 98 ± 4 | 96 ± 1 | 104 ± 6 | 90 ± 5 | 94 ± 9 |
III. Dialysis profile
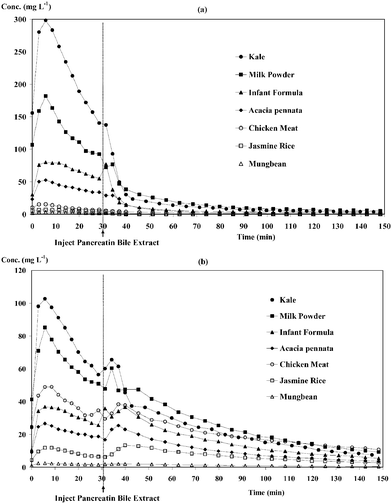
Not only was the percent dialysability obtained but also the dialysis profiles of elements and pH change profiles, which can be useful in understanding the dialysis change and factors affecting dialysability. The pH changes (right axis of Fig. 2) from approximately 2.0 for gastric digests to ca. 5.0 within 30 min of intestinal digestion and to ca. 7.0–7.5 after 1 h were close to what occurs in the human gastrointestinal tract.4 The blank dialysis (Fig. 2) was found to give low values (<4 mg L−1 for all elements, highest for P at 3–7 mg L−1, which was considerably low and could be estimated to be less than 10% of the dialysable components). Nevertheless, blank correction was performed and subtracted in all analyses. Figs. 3(a) and 3(b) show dialysis profiles of Ca, Mg, P, Fe and Zn for milk powder and kale digests, respectively. All elements show similar profiles, giving peak maxima at about 10 min of dialysis and a gradual decrease to baseline; in this system, such profiles equate to “absorption”, seen here as loss of mineral from the model intestine. The irregular pH change and dialysis profiles at 30–40 min were probably affected by PBE injection at 30 min.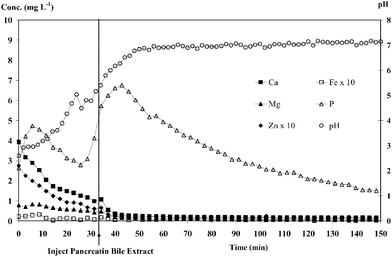 | ||
| Fig. 2 Dialysis profiles and pH changes obtained from the CFD-pH-ICP-OES system for blank analysis. | ||
 | ||
| Fig. 3 Dialysis profiles and pH changes in milk powder (a) and kale (b). | ||
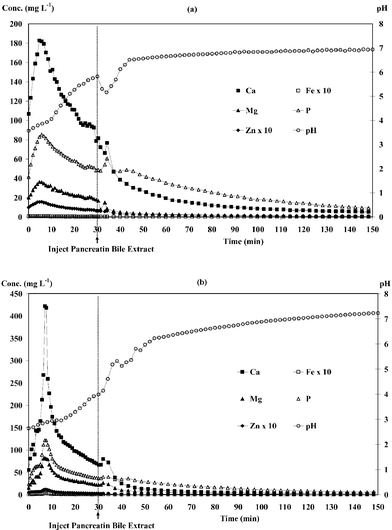
Fig. 4 shows dialysis profiles of Ca (a) and P (b) in different food digests. Calcium and phosphorus in kale and milk powder were rapidly dialysed in the first 30 min. It was found that P dialysis profiles showed a second peak after PBE injection, as illustrated in Fig. 4(b), as a result of digestion of food components by PBE enzymes. The descending order of the total calcium content (Table 3) is as kale > milk powder > infant formula > Acacia pennata > chicken meat > jasmine rice > mungbean (ranged from 13![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 867 to 56 μg g−1) considering their dialysed calcium.
867 to 56 μg g−1) considering their dialysed calcium.
| Jasmine rice | Chicken meat | Mungbean | Acacia pennata | Milk powder | Kale | |
|---|---|---|---|---|---|---|
| Calcium | ||||||
| Total minerals/μg g−1 | 56 ± 3 | 299 ± 13 | 819 ± 43 | 1650 ± 30 | 6890 ± 120 | 13![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 870 ± 540 870 ± 540 |
| Dialysed minerals/μg g−1 | 44 ± 2 | 279 ± 9 | 714 ± 35 | 1220 ± 55 | 5560 ± 280 | 8520 ± 430 |
| Non-dialysed minerals/μg g−1 | 8 ± 2 | 12 ± 5 | 119 ± 27 | 482 ± 25 | 1030 ± 30 | 5520 ± 240 |
| Dialysed + non-dialysed minerals/μg g−1 | 52 ± 4 | 290 ± 13 | 842 ± 26 | 1760 ± 91 | 6580 ± 280 | 14![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 030 ± 520 030 ± 520 |
| Dialysis (%) | 78 ± 3 | 93 ± 3 | 87 ± 4 | 74 ± 3 | 81 ± 4 | 61 ± 3 |
| Element retained (%) | 14 ± 3 | 4 ± 2 | 15 ± 3 | 31 ± 2 | 15 ± 0.5 | 40 ± 2 |
| Sum (%) | 94 ± 6 | 100 ± 4 | 103 ± 3 | 105 ± 6 | 96 ± 4 | 101 ± 4 |
| Magnesium | ||||||
| Total minerals/μg g−1 | 157 ± 3 | 878 ± 30 | 1410 ± 3 | 1980 ± 12 | 763 ± 29 | 4690 ± 310 |
| Dialysed minerals/μg g−1 | 95 ± 4 | 814 ± 13 | 1050 ± 40 | 1640 ± 44 | 628 ± 46 | 3130 ± 100 |
| Non-dialysed minerals/μg g−1 | 34 ± 6 | 47 ± 2 | 459 ± 3 | 417 ± 7 | 69 ± 3 | 1470 ± 40 |
| Dialysed + non-dialysed minerals/μg g−1 | 129 ± 3 | 861 ± 14 | 1507 ± 43 | 2080 ± 50 | 697 ± 45 | 4600 ± 90 |
| Dialysis (%) | 60 ± 2 | 93 ± 2 | 74 ± 3 | 84 ± 2 | 82 ± 6 | 67 ± 2 |
| Element retained (%) | 22 ± 4 | 5 ± 0.2 | 33 ± 0.2 | 21 ± 1 | 9 ± 0.4 | 31 ± 1 |
| Sum (%) | 82 ± 2 | 98 ± 2 | 107 ± 3 | 105 ± 2 | 91 ± 6 | 98 ± 2 |
| Phosphorus | ||||||
| Total minerals/μg g−1 | 839 ± 41 | 6060 ± 209 | 4000 ± 60 | 8540 ± 220 | 6610 ± 310 | 5990 ± 60 |
| Dialysed minerals/μg g−1 | 77 ± 8 | 2720 ± 90 | 525 ± 13 | 3250 ± 120 | 3680 ± 180 | 3240 ± 210 |
| Non-dialysed minerals/μg g−1 | 699 ± 26 | 2490 ± 340 | 3750 ± 40 | 5390 ± 260 | 1610 ± 30 | 1840 ± 40 |
| dialysed + non-dialysed minerals/μg g−1 | 776 ± 25 | 5210 ± 370 | 4280 ± 60 | 8630 ± 340 | 5160 ± 140 | 5280 ± 60 |
| Dialysis (%) | 9 ± 1 | 45 ± 1 | 13 ± 0.3 | 38 ± 1 | 56 ± 3 | 54 ± 3 |
| Element retained (%) | 83 ± 3 | 45 ± 1 | 94 ± 1 | 63 ± 3 | 23 ± 2 | 31 ± 1 |
| Sum (%) | 92 ± 3 | 90 ± 2 | 107 ± 1 | 101 ± 4 | 79 ± 2 | 88 ± 1 |
| Iron | ||||||
| Total minerals/μg g−1 | 3.8 ± 0.1 | 15.2 ± 0.8 | 46.8 ± 2.1 | 113.4 ± 10.2 | 69.0 ± 1.4 | 90.9 ± 8.6 |
| dialysed minerals/μg g−1 | 0.2 ± 0.1 | 0.7 ± 0.2 | 2.2 ± 0.3 | 11.8 ± 0.9 | 3.7 ± 0.7 | 4.8 ± 0.4 |
| Non-dialysed minerals/μg g−1 | 3.2 ± 0.1 | 14.0 ± 0.3 | 41.4 ± 1.4 | 104.9 ± 1.9 | 55.5 ± 3.1 | 85.5 ± 2.8 |
| dialysed + non-dialysed minerals/μg g−1 | 3.5 ± 0.1 | 14.7 ± 0.4 | 43.6 ± 1.4 | 116.7 ± 2.7 | 59.2 ± 3.4 | 90.4 ± 2.6 |
| Dialysis (%) | 6 ± 1 | 5 ± 1 | 5 ± 1 | 10 ± 1 | 5 ± 1 | 5 ± 1 |
| Element retained (%) | 84 ± 3 | 92 ± 2 | 89 ± 3 | 92 ± 2 | 80 ± 4 | 94 ± 3 |
| Sum (%) | 91 ± 3 | 96 ± 2 | 93 ± 3 | 103 ± 2 | 86 ± 5 | 100 ± 3 |
| Zinc | ||||||
| Total minerals/μg g−1 | 19.4 ± 0.6 | 27.5 ± 1.8 | 29.6 ± 0.4 | 44.0 ± 3.2 | 50.7 ± 2.3 | 32.0 ± 4.0 |
| Dialysed minerals/μg g−1 | 10.8 ± 1.3 | 22.9 ± 0.7 | 21.3 ± 0.2 | 15.9 ± 0.6 | 38.3 ± 1.7 | 18.2 ± 0.9 |
| Non-dialysed minerals/μg g−1 | 6.5 ± 0.7 | 6.9 ± 0.2 | 10.4 ± 0.8 | 22.9 ± 0.8 | 7.2 ± 0.1 | 15.1 ± 0.8 |
| Dialysed + non-dialysed minerals/μg g−1 | 17.3 ± 1.4 | 29.8 ± 0.7 | 31.5 ± 0.6 | 38.8 ± 0.6 | 44.3 ± 1.1 | 32.6 ± 0.8 |
| Dialysis (%) | 55 ± 7 | 83 ± 2 | 72 ± 1 | 36 ± 1 | 76 ± 3 | 57 ± 3 |
| Element retained (%) | 33 ± 4 | 25 ± 1 | 35 ± 3 | 52 ± 2 | 14 ± 0.2 | 47 ± 2 |
| Sum (%) | 89 ± 7 | 108 ± 3 | 106 ± 2 | 88 ± 2 | 87 ± 2 | 102 ± 3 |
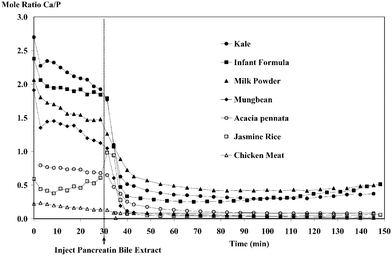
IV. Profile of molar ratio plot
From a nutritional point of view, the ratio of Ca and P intake is believed to be important: the optimum molar ratio of Ca/P is 1, although a Ca/P ratio of 1/1.5 is acceptable. Injurious effects can appear when this relationship is not met.14 It is important to maintain a good ratio of Ca and P intake for optimal bone mineralization.15 Anderson,16 in a review of the relationship of Ca and P and human bone development, stated that a potential mechanism for the development of low bone mass in the United States was related to a low ratio of Ca/P intake. Consumed diets with low Ca in dairy foods can also potentially contribute to a low Ca/P ratio.17 Wyatt and co-workers18 found that beans and corn tortillas were the main contributors of Ca and P in the diet, contributing higher levels of P than Ca, which resulted in Ca/P of 1 ∶ 1.8.From the Ca and P levels found in the dialysed samples, the Ca/P ratio was calculated. The molar ratio plot of Ca/P in various kinds of food is markedly different (see Fig. 5). The ratio of Ca/P of dialysed amount is higher than that of the total mineral content in the same food (Table 4). The molar ratio of Ca/P from total mineral content in milk powder is 0.81 in this study, which is similar to an earlier report.19 In contrast, the Ca/P ratio from total mineral content in kale is 1.80, which differs from the values in an earlier study20 of 2.44–3.01, possibly owing to the fact that kales were from different geographical areas and might be of different species. However, this ratio for dialysed amount is, in all cases, higher because Ca gives higher % dialysis than P in all food samples (see Table 3). Although the Ca/P is important in determining their biological activity, this does not extend to absorption. Nonetheless, the study of the molar ratio profile might be of potential use in the future.
V. Application of the proposed system to estimate mineral dialysability of various foods
Dialysability was calculated by the summation of dialysed amounts of mineral during intestinal digestion. The results from the determination of the dialysability of essential elements from different kinds of food by the proposed method are shown in Table 3. Total Ca content was low in jasmine rice (56 ± 3 μg g−1) and high in kale (13![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 870 ± 540 μg g−1). The percentage of dialysis in all samples ranged from 61 to 93%, the percentage of Ca remained ranged from 4 to 40% and the sum of these values ranged from 94 to 105%. Magnesium content in studied foods ranged from 157 ± 3 μg g−1 in jasmine rice to 4690 ± 310 μg g−1in kale. The percentage of dialysis varied from 60% in jasmine rice and 93% in chicken meat. Percent Mg remaining ranged from 5 to 33% and the sum of these values ranged from 82 to 105%. Most studied foods contained high amounts of total P (more than 2490 μg g−1) except that of jasmine rice (839 μg g−1). The percent dialysis of P was lower than 56% and the sum of percent dialysis and percent remained minerals was 79–107%. The percent dialysis of Fe was lowest at 5–10%, and the recovery was 86–103%. For Zn, the summation of dialysed and remaining Zn was also acceptable (87–108%).
870 ± 540 μg g−1). The percentage of dialysis in all samples ranged from 61 to 93%, the percentage of Ca remained ranged from 4 to 40% and the sum of these values ranged from 94 to 105%. Magnesium content in studied foods ranged from 157 ± 3 μg g−1 in jasmine rice to 4690 ± 310 μg g−1in kale. The percentage of dialysis varied from 60% in jasmine rice and 93% in chicken meat. Percent Mg remaining ranged from 5 to 33% and the sum of these values ranged from 82 to 105%. Most studied foods contained high amounts of total P (more than 2490 μg g−1) except that of jasmine rice (839 μg g−1). The percent dialysis of P was lower than 56% and the sum of percent dialysis and percent remained minerals was 79–107%. The percent dialysis of Fe was lowest at 5–10%, and the recovery was 86–103%. For Zn, the summation of dialysed and remaining Zn was also acceptable (87–108%).
In summary, total element and percent dialysability of each element is markedly influenced by the nature of the food. Kale and milk powder contained high total amounts of Ca, Mg, P, and Zn. Chicken meat contained a high total Fe content, whereas mungbean was high in total Mg content. The dialysabilities of Ca, Mg, and Zn are relatively high (36–93%). All studied foods had varying P dialysability, 9 ± 1% in jasmine rice and 56 ± 3% in milk powder. In contrast, the Fe dialysability is lowest at 5–10%.
Conclusions
The developed CFD-pH-ICP-OES system is simple, rapid and capable of continuous multielement detection. The system can be used for simultaneous monitoring of dialysed minerals concentration and pH during dialysis. The developed system was successfully applied to estimate the essential minerals dialysability of various kinds of foods. Information about minerals dialysability can be useful for the nutritional evaluation of foods and for the study of the effect of food components on mineral bioavailability. The dialysis profiles of elements and pH change profiles can be useful to understand the dialysis change and factors affecting dialysability. All studied elements were rapidly dialysed in the first 30 min of simulated intestinal dialysis. Not only was the profile of dialysed minerals obtained but also the molar ratio profiles of elements, which can be useful for nutritional evaluation of foods. Knowledge of mineral bioavailability is useful for managing mineral intake and for reduction of the health risk from mineral deficiency.Acknowledgements
The authors are grateful for financial support from the Thailand Research Fund and the Postgraduate Education and Research Program in Chemistry, Higher Education Development Project, Ministry of Education.References
- K. Van Dyck, S. Tas, H. Robberecht and H. Deelstra, Int. J. Food Sci. Nutr., 1996, 47, 499–506 CrossRef CAS.
- M. Hansen, B. Sandstrom and B. Lonnerdal, Pediatr. Res., 1996, 40(4), 547–552 Search PubMed.
- D. D. Miller, B. R. Schricker, B. S. Rasmussen and D. Van Campen, Am. J. Clin. Nutr., 1981, 34, 2248–2556 CAS.
- M. G. E. Wolters, H. A. W. Schreuder, G. Van Den Heuvel, H. J. Van Lonkhuijsen, R. J. J. Hermus and A. G. J. Voragen, Br. J. Nutr., 1993, 69, 849–861 CrossRef CAS.
- L. H. Shen, J. Luten, H. Robberecht, J. Bindels and H. Deelstra, Z. Lebensm. Unters. Forsch., 1994, 199, 442–445 CrossRef CAS.
- J. Shiowatana, W. Kitthikhun, U. Sottimai, J. Promchan and K. Kunajiraporn, Talanta, 2005 Search PubMed , in the press; DOI: 10.1016/j.talanta.2005.04.068.
- M. J. Roig, A. Alegria, R. Barbera and M. J. Lagarda, Food Chem., 1999, 65, 353–357 CrossRef CAS.
- M. Santaella, I. Martinez, G. Ros and J. Periago, Meat Sci., 1997, 45, 473–483 CrossRef CAS.
- M. Lucarini, R. Canali, M. Cappelloni, G. Di Lullo and G. Lombardi-Boccia, Food Chem., 1999, 64, 519–523 CrossRef CAS.
- K. J. H. Wienk, J. J. M. Marx and A. C. Beynen, Eur. J. Nutr., 1999, 38, 51–75 CrossRef CAS.
- J. Promchan and J. Shiowatana, Anal. Bioanal. Chem., 2005, 382, 1360–1367 CrossRef CAS.
- J. M. Pettifor, ‘Rickets’, in Dietary Calcium Deficiency, ed. F. H. Glorieux, Raven Press, New York, 1991, pp. 123–143 Search PubMed.
- F. Cámara, M. A. Amaro, R. Barberá and G. Clemente, Food Chem., 2004, 92, 481–489.
- P. Aranda and J. Llopis in Nutrition Dietetic: Aspects Sanitarios, Consejo General de Colegios Oficiales de Farmaceuticos, Madrid, 1993, pp. 183–233 Search PubMed.
- I. Martinez, M. Santaella, G. Ros and J. Periago, Food Chem., 1998, 63(3), 299–305 CrossRef CAS.
- J. J. B. Anderson, J. Nutr., 1996, 126, 1153s–1158s CAS.
- J. J. Barger-Lux, R. P. Heaney, P. T. Packard, J. M. Lappe and R. R. Reeker, Clin. Appl. Nutr., 1992, 2, 39–45 Search PubMed.
- C. J. Wyatt, M. E. Hemandez-Lozano, R. O. Mendez and M. E. Valencia, Nutr. Res., 2000, 20(3), 427–437 CrossRef CAS.
- A. Lante, G. Lomolino, M. Cagnin and P. Spettoli, Food Control, 2004 Search PubMed , in the press (DOI:10.1016/j.foodcont.2004.10.010).
- M. Umetaa, C. E. West and H. Fufaa, J. Food Compos. Anal., 2005, 18, 803–817 CrossRef.
Footnote |
| † Presented at the First Winter Conference on Plasma Spectrochemistry, Chiang Mai, Thailand, April 25–30, 2005. |
| This journal is © The Royal Society of Chemistry 2005 |