Wax esters produced by solvent-free energy-efficient enzymatic synthesis and their applicability as wood coatings
Anna E. V.
Petersson
*a,
Linda M.
Gustafsson
b,
Mathias
Nordblad
a,
Pål
Börjesson
b,
Bo
Mattiasson
a and
Patrick
Adlercreutz
a
aDepartment of Biotechnology, Center for Chemistry and Chemical Engineering, Lund University, P.O. Box 124, SE-221 00, Lund, Sweden. E-mail: anna.petersson@biotek.lu.se
bEnvironmental and Energy Systems Studies, Department of Technology and Society, Lund University, Gerdagatan 13, SE-223 62, Lund, Sweden
First published on 7th November 2005
Abstract
The study aimed at developing a process for making a wood coating wax based on the principles of green chemistry. The research was conducted within the Swedish interdisciplinary research programme Greenchem. Wax esters are attractive since they are non-hazardous, biodegradable and can be produced in an atom-efficient process from building blocks obtained from renewable resources. Four wax esters were prepared in a solvent-free process using an immobilised lipase as catalyst. When the water was removed during the process from what was initially an equimolar mixture of the starting materials carboxylic acid and alcohol by a stream of dry air passed through the reactor, there was a 95–99% conversion to the ester. The enzymatic process consumed 34% less energy and generated less waste than chemical esterification using a strong acid as catalyst. Two of the esters worked well in the industrial wood coating equipment employed and produced surfaces resistant to water and somewhat less to fat stains.
Introduction
Industrial interest in biotransformation processes, long a subject of research within academia, has been increasing recently. This has been shown by the increasing number of industrial-scale bioprocesses started during the last decade1 and by the chemical industry's exploring possibilities of using renewable resources to replace crude oil and improve sustainability.2 Two factors contributing to the expanding interest in this area have been the desire to utilise more environmentally benign processes and the fact that crude oil is a finite resource that there eventually will be a shortage of, with an accompanying of increasing costs. Most of today's commercial enzymatic processes possess a variety of positive characteristics, such as high productivity, high product concentrations, and a lack of undesirable by-products,3 characteristics particularly evident in certain enzymatic processes, such as in esterification catalysed by lipases, which typically gives clean products with little need of downstream processing.The research programme “Speciality Chemicals from Renewable Resources—Greenchem” is a Swedish interdisciplinary research programme concerned with the development and application of biocatalysts for the production of fine chemical products from renewable raw materials. The programme includes research activities within both biotechnology and environmental systems analysis and involves cooperation with several industrial partners.
Consumer and industrial desire for more environmentally benign paints and coatings is growing rapidly.4 There is a strong interest here in developing waxes to serve as ingredients in coatings for wooden surfaces, with a minimum of pollutants and with substrates from renewable resources. The use of wax esters (esters of long-chain carboxylic acids and long-chain alcohols) is attractive since these are non-hazardous compounds with good biodegradability. Wax esters can be made from renewable feedstocks, such as vegetable oils. High atom economy can also be achieved, one molecule of water being the only side-product in the key production step, that of condensation of the carboxylic acid and the alcohol. The reaction can be carried out catalytically, either using a chemical catalyst such as a strong acid or using an enzyme. The enzymatic process presented in this work thus fulfils several of the twelve principles of green chemistry formulated by Anastas and Warner in 1998.5 A conceptual picture of our work can be seen in Fig. 1. In the present study we make a theoretical comparison of the chemical and the enzymatic alternatives from a green chemistry perspective.
 | ||
| Fig. 1 Illustration of wax production from renewable resources. | ||
Life cycle assessment (LCA) has been recognised as a tool for the environmental evaluation of new, green alternative processes. This involves quantifying the benefits these have compared to the traditional chemical processes. In an ordinary LCA, the total environmental impact is calculated for the complete life cycle of the product, from cradle-to-grave. Such LCAs include feedstock production and the manufacturing, use and final disposal of the product. In considering only the processes, a gate-to-gate perspective can be employed. This is relevant when the systems compared utilise the same raw material and the product they result in are the same. One important parameter of LCA is the input of energy, which is analysed here for the processes involved.
A schematic diagram of the chemical and the enzymatic processes is shown in Fig. 2. Because of the advantages of the enzymatic processes, the laboratory work was concentrated on this alternative.
 | ||
| Fig. 2 Schematic diagrams of conventional chemical ester synthesis and the corresponding enzymatic process.26 | ||
In efforts to develop an attractive, green process for the enzymatic production of wax esters, key points to address were the evaluation of possibilities for use of a solvent-free process and of finding methods for achieving sufficiently high conversion levels to allow the product to be used with only a minimum of purification. From an economic point of view, the process had to be efficient enough to prevent the enzyme costs from becoming prohibitive. To this end, a particular process methodology was developed, one allowing four different wax esters to be produced enzymatically in a litre-scale reactor. Their properties as wood coating waxes were investigated by the industrial partners that participated.
Results and discussion
Choice of the solvent to be used in a process is a key issue, from a green chemistry perspective. Supercritical carbon dioxide and ionic liquids are often referred to as “green solvents”, but use of a solvent-free process is the most attractive alternative. Solvent-free enzymatic esterification processes, having been thoroughly described previously, were a natural choice in the present study. The solvent-free synthesis of cetyl palmitate was performed in a 25 ml round-bottomed flask. Two different methods were used for monitoring the reaction, the one being analysis of cetyl alcohol by GC and the other titration of the acid groups. These methods produced similar results. A conversion of more than 98% of the cetyl alcohol was achieved.Since esterification reactions are reversible, it is advantageous, in order to achieve a high ester yield, that the reaction mixture contain as little water as possible. In concentrated systems, such as solvent-free reaction mixtures, water formed by the reaction should preferably be removed. Several means of water removal have been suggested, such as vacuum evaporation,6 pervaporation,7,8 addition of cation-exchange resins,9 use of molecular sieves10,11 and azeotropic distillation.12 Some enzymes need a certain amount of water in order to display acceptable catalytic activity. In such cases, water removal at a fixed water activity (relative humidity) is better than extensive drying. Methods to control the water activity of the reaction mixture involve use of salt hydrate pairs13–15 or saturated salt solutions, either for pre-equilibration13,16 or for continuous control.17–20 These alternatives have mainly been used in small-scale synthesis. Use of a vacuum for drying purposes has been studied extensively and is also applicable on a large scale. It has been shown that the water activity can be controlled by air-bleeding in a vacuum reactor,6 although this method requires rather complicated equipment. A practical way of controlling the water activity in a reactor is to measure it continuously with a sensor and to adjust it to a set value by passing air (or nitrogen) through the reaction mixture. Dry air is used to remove water from the reactor and humid air to add it.21–24 This can be carried out on a small and a large scale. If needed, the water activity can also be set at different values for separate stages of the reaction. A further advantage of this approach is that the gas bubbles produced improve the mixing in the reactor so that stirring can be reduced or even be avoided entirely, lowering the energy consumption.22 This method has the drawback, however, that volatile substrates and products can be lost in the gas stream, although this is not of major concern when dealing with non-volatile substances, such as in the present study. For the synthesis of wax esters, a one litre reactor containing a water activity sensor and equipment for the automatic adjustment of water activity by use of dry or humid air was constructed (Fig. 3).
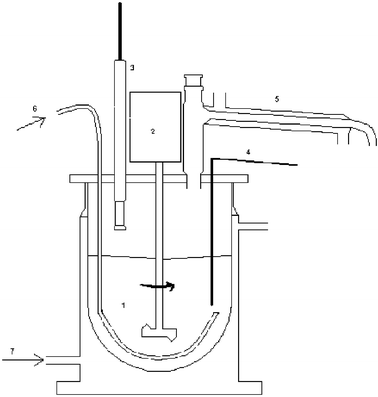 | ||
| Fig. 3 Litre scale reactor for enzymatic esterification. 1—reaction liquid, 2—stirrer, 3—water activity sensor, 4—thermometer, 5—condenser, 6—dry air inlet, 7—heating medium, glycerol, inlet. | ||
In the 25 ml-scale, removal of water occurred by simple evaporation to the surrounding air, but when the synthesis of cetyl palmitate was performed in the litre-scale reactor without any deliberate water removal, only ∼65% conversion was obtained (Fig. 4). Thereafter, drying by the passing of dry air through the reactor was started and ∼95% conversion was achieved. When the reaction mixture was dried by use of dry air from the start, almost complete conversion to cetyl palmitate was achieved (Fig. 5). Similar levels of conversion for this reaction have been reported using water activity adjustment involving the pumping of saturated salt solutions through silicone tubing in the reactor.17 However, this method has certain drawbacks, such as the relatively slow transfer of water between the two phases and the limited suitability of it for large-scale processes.
 | ||
| Fig. 4 Conversion (■) and water activity during the reaction of cetyl alcohol and palmitic acid 1 ∶ 1, using Novozym® 435 as a catalyst, 0.5 g mol−1 substrate. The enzyme was added at time 0 and drying started after 29.5 h. | ||
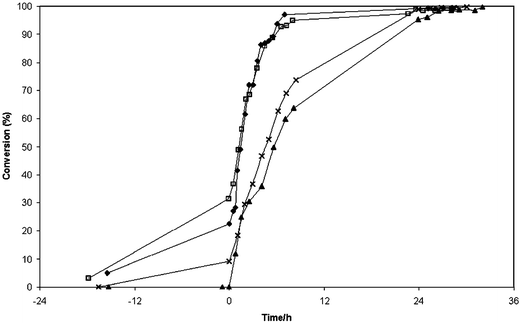 | ||
| Fig. 5 Comparisons of the conversion levels achieved for the four wax esters produced: cetyl palmitate (◆), behenyl behenate (□), dibehenyl adipate (▲) and dibehenyl sebacate (×). The syntheses were performed using equimolar amounts of the reactive groups catalysed by Novozym® 435, 1 g mol−1 reactive group. The substrates were melted and dried before the enzyme was added at time 0. The reaction temperatures are listed in Table 2. | ||
Two monoesters, cetyl palmitate and behenyl behenate, and two diesters, dibehenyl adipate and dibehenyl sebacate, were produced (Tables 1 and 2) in the one litre reactor. The reaction temperatures were chosen so that all the substrates (monoester synthesis) or a major part of them (diester synthesis) were in liquid form. An increase in temperature leads to an increase in reaction rates, but also to an increase in enzyme inactivation rates as well as an increase of the energy consumption.
| Cetyl palmitate | H3C(CH2)15OOC(CH2)14CH3 |
| Behenyl behenate | H3C(CH2)21OOC(CH2)20CH3 |
| Dibehenyl adipate | H3C(CH2)21OOC(CH2)4COO(CH2)21CH3 |
| Dibehenyl sebacate | H3C(CH2)21OOC(CH2)8COO(CH2)21CH3 |
| Ester | Temperature/°C | Initial reaction rate/mmol min−1 g−1 | Melting point product/°C | Final conversion of | |
|---|---|---|---|---|---|
| alcohol (%) | acid (%) | ||||
| Cetyl palmitate | 65–67 | 3.4 | 50–51 | 98 | 99 |
| Behenyl behenate | 85–88 | 2.9 | 69–73 | 99 | 98 |
| Dibehenyl adipate | 90–93 | 1.4 | 70–73 | 99 | 99 |
| Dibehenyl sebacate | 88–90 | 1.4 | 71–74 | 95 | 99 |
All four esters were synthesised at high conversions, see Table 2. In most cases, as can be seen in Fig. 4 and 5, some conversion could be observed before addition of enzyme, which could be explained by spontaneous esterification during the drying of the substrates. The various reactions differed in initial reaction rate after enzyme was added, as can be seen in Fig. 5. The initial reaction rate was highest for cetyl palmitate, despite this reaction being run at a lower temperature than the others. It has been shown earlier that between 65 and 75 °C the reaction rate for isopropyl palmitate synthesis increases with increasing temperature.25 According to the manufacturer, the optimal temperature of this immobilised enzyme is 70–80 °C. The initial reaction rates for the monoesters were approximately twice as high as those for the diesters, dibehenyl adipate and dibehenyl sebacate. This might possibly be due to the fact that at the reaction temperature employed the diacids were not completely melted or dissolved initially. As the reactions proceeded, they dissolved, the reaction mixtures then becoming clear.
The energy requirements for the large-scale production of wax esters (25 tonnes annually) were estimated, both for batch and for continuous reactor systems using two different types of water removal systems: air-stripping or evaporation. In addition, estimates were made of the energy requirements of a conventional process. The results of the calculations on the best enzymatic and the conventional alternative are shown in detail in Table 3. The reactor system most similar to the reactor used in the laboratory-scale experiments described in the present study is a batch reactor employing air stripping. There are certain differences between the laboratory- and the large-scale systems, however, in large-scale production there are separate heating of the ingoing air and the reactor, and drying by heated air from the surroundings rather than by dried air and heat exchange between the in- and outgoing air. These differences contribute to increasing the energy efficiency of large-scale production.
The energy requirements for the large-scale reactor systems vary between 500 and 1500 MJ tonne−1, the different batch reactors being the most efficient. On the basis of calculations concerning the best enzymatic process (batch reactor), the conventional method had an estimated energy requirement of 765 MJ tonne−1, which is one and a half times that of the enzymatic process. The preheating of the substrates is the most energy-consuming step in the process, representing 60% (300 MJ tonne−1) of the total energy requirement for the enzymatic process. For a chemical process conducted at 150 °C, the preheating step would require 70% (540 MJ tonne−1) of the total energy requirements, which is 80% greater than for the enzymatic process. Several post-production steps are needed for the conventional method, although these have not been included in the calculations. Calculations performed by Hills26 show that the chemical method, due to the higher temperature and the post-reaction purification steps, has energy requirements up to two and a half times as high as for enzymatic production.
Measurements of the litre-scale synthesis of wax esters indicate energy requirements of 125–160 GJ tonne−1 of the product, depending on the wax ester involved. The large difference as compared with the calculations for large-scale production (two orders of magnitude, in fact) clearly demonstrates the need of adequate insulation and energy recirculation for an acceptable level of energy efficiency to be achieved.
The large-scale enzymatic production of wax esters commercially is still under development, and there are only few companies utilising these reactions, Degussa being one. The enzymatic process has several advantages over the conventional chemical methods, which usually involve use of temperatures above 150 °C, the reaction thus being unselective and the waste and catalyst residues needing to be removed in post-reaction purification. According to Hills, the amount of waste (such as solvents, bleaching residues and by-products) generated in conventional chemical production is up to five times as high as in enzymatic production. In addition, for the chemical methods neutralisation of the acid, steam treatment for distillation, and both deodorizing and bleaching are needed, steps that involve use of a strong acid, and an alkaline or an acid, together with a catalyst. For the chemical method, drying of the product is also needed, as well as larger amounts of raw materials due to the unselectiveness of the process. During the post-reaction stages there is a loss in product yield as well, up to five percent according to Hills.26 Martinez27 has performed studies similar to Hills'. She has found that the enzymatic production of wax esters is more environmentally benign than the conventional chemical one. The lesser amount of refining needed also results in higher yield. Products made with use of such enzymatic reactions have a higher consumer appeal than those made using the conventional processes, due to the environmental benefits that result. The presented study yielded results similar to Hills' in showing energy use and waste to be less in the enzymatic processes.
The wax esters were emulsified in water using a surfactant, the emulsions being treated further to allow them to be used in industrial wood coating equipment. The monoesters cetyl palmitate and behenyl behenate behaved well in the wood coating equipment and produced waxy surfaces on pine wood, whereas the diesters formed precipitates in the coating equipment, making further evaluation of them impossible. Since the melting points of the diesters were only slightly higher than the melting point of the behenyl behenate, it was probably some other property of the diesters that caused this behaviour. The monoesters produced surfaces with good resistance to water, almost as good as the existing product, Vaxoline (Table 4). However, the resistance to fat achieved was relatively poor. In order to improve the fat resistance, it is likely that the size of the molecules needs to be increased. Work in this direction is continuing with polyesters. Additionally more additives may be needed to gain the desired properties. The wax esters produced in the study will be evaluated for other applications as well.
| Vaxoline | Cetyl palmitate | Behenyl behenate | Dibehenyl adipate | Dibehenyl sebacate | |
|---|---|---|---|---|---|
| Performance in coating equipment | 5 | 5 | 5 | 1 | 1 |
| Water 16 h | 5 | 4 | 4 | n/a | n/a |
| Fat 6 h | 5 | 2 | 3 | n/a | n/a |
| Fat 24 h | 4 | 1 | 2 | n/a | n/a |
Experimental
Enzymatic solvent-free synthesis of wax esters
Chemicals and enzyme preparation
Cetyl alcohol (96%), palmitic acid (98%), adipic acid (>99%) and sebacic acid (>95%) were purchased from Sigma Aldrich. The following chemicals were generously donated by the companies referred to: behenyl alcohol (80–85%), Cognis (Boussens, France); behenic acid (85–90%), Croda Chemicals (East Yorkshire, England); Novozym® 435 (immobilised Candida antarctica lipase B), Novozymes A/S (Bagsværd, Denmark); glycerol, Karlshamns AB (Karlshamn, Sweden); and the surfactant, Bermodol 2525, Akzo Nobel Surfactants AB (Stenungsund, Sweden). All the other chemicals were of analytical grade.Small-scale enzymatic synthesis of cetyl palmitate
Small scale synthesis of cetyl palmitate was performed at 65 °C in a three-necked round-bottomed flask. The alcohol and the acid were mixed in equimolar amounts and, when the substrates had been melted, an immobilised enzyme, Novozym® 435, was added to start the reaction. During the reaction, water activity and temperature were measured in the air above the reaction liquid by a relative humidity sensor, Hygroflex 3 (Rotronic AG, Basseldorf, Germany). The reaction mixture was analysed by gas chromatography.Litre-scale enzymatic synthesis of wax esters
Litre-scale synthesis of the wax esters was performed in a jacketed glass reactor using glycerol as the heating medium, see Fig. 3. The reaction was carried out at elevated temperatures, the exact temperature depending upon the product to be synthesised. To dry the reaction, air was bubbled through two gas-washing bottles containing blue silica gel, before it was sent to the reactor through small holes in narrow steel tubes. During the reaction, the water activity and the temperature were measured by a sensor, Hygroflex 3 (Rotronic AG, Basseldorf, Germany), which was present in the air above the reaction liquid. The reaction was followed by gas chromatography and/or titration of the remaining acid. Final samples were also analysed by titration of the hydroxyl groups. Following the reaction, the enzyme can be filtered from the product and be reused, approximately 5–6 times altogether.Gas chromatography
Wax ester production was analysed by a gas chromatography device (GC-14A, Shimadzu Corp., Kyoto Japan) equipped with a flame-ionisation detector and a capillary column, DB5 (Supelco SPB5 15 m, 0.32 mm i.d., 25 μm film), purchased from Sigma Aldrich. Helium served as the carrier gas. The temperature of the injector and the detector was 350 °C. A temperature program between 225 °C and 320 °C was employed. Samples, taken by weight (20–90 mg), were diluted (×1000) in cyclohexane:methyl ethyl ketone (11 ∶ 1).Titration of acid groups
A sample was dissolved in a cyclohexane ∶ methyl ethyl ketone mixture (11 ∶ 1), 20 drops phenolphthalein (1% in ethanol) being added. The solution was then titrated with a KOH solution in ethanol until a shift in colour was achieved. The amount of acid left was calculated in accordance with the equimolar relationship between the amount of potassium hydroxide added and the amount of acid in the sample.Titration of hydroxyl groups
A sample was dissolved in 40 ml tetrahydrofuran, 10 ml of 4-dimethylaminopyridine solution (2.5% in acetone) being added, the mixture being stirred for one minute, and 2 ml of acetic acid anhydride solution (25% in methyl ethyl ketone) being added. The sample was stirred then for 5–10 min, after which 30 drops of phenolphthalein (1% in ethanol) were added. This solution was titrated with 1 M KOH in water until a colour shift was achieved. The amount of alcohol in the sample was calculated on the basis of the difference in the amount of potassium hydroxide between the sample and a blank, i.e. a titrated solution without the sample.Emulsification of wax esters and application and evaluation as a wood coating ingredient
The coating in which the wax esters were tested is water based, with a dry content of approximately 20%. The commercial Vaxoline product contains an acrylate in combination with a wax emulsion, whereof approximately 40% of the dry weight is acrylate and the rest is wax. The wax ester tested was melted and 150 ml of it was mixed with 330 ml water and 20 ml Bermodol SPS 2525 using a homogeniser, Yellowline DI 25 basic, IKA (Staufen, Germany) to get an emulsion.The wax ester emulsion was then mixed with small amounts of additives, and sprayed on solid glued samples of planed Scots pine, 8 × 200 × 250 mm in size. The emulsions were applied in one layer, 70 g m−2, and then dried at 40 °C overnight.
Assessment of the surface resistance to water and paraffin oil (fat) was performed according to EN 12720 (European Standard EN 12720 (1997) Furniture—Assessment of surface resistance to cold liquids), a filter paper immersed in a liquid, being placed on the test surface and being covered by an inverted glass basin. After a specified period of time, the paper was removed and the surface was washed and dried. The surface was then examined for possible damages such as discolouration, change in gloss or colour, blistering etc. The assessment of the test results was performed in terms of a descriptive numerical rating code.
Energy analysis—a comparison between conventional chemical process and enzymatic production
The product investigated is a wax ester produced, in the first alternative, by conventional chemical method, and in the second alternative, by biocatalysis using enzymes.Enzymatic process—biocatalytic synthesis of wax ester
Calculations for a large-scale production facility have been conducted for a total of six reactor systems, using air-stripping or evaporation for water removal. Heating of the reactor was achieved by circulating hot water in a mantle around the reactor and/or by preheating the air used for water removal. Both batch and continuously stirred tank reactors were evaluated. The calculations were based on suitable reactor volumes for an annual productivity of 25 tonnes, which were estimated to be 102 L for batch and 22 L for the continuous systems. For the reactor 20 mm of insulation was used and a heat exchanger was assumed for in- and outgoing air flows when applicable, with an efficiency of 83%. The efficiency of the air heating was assumed to be 90%. The ambient temperature was assumed to be 20 °C in all cases. In addition to the calculations on large-scale processes, measurements were made on the energy requirements for the litre-scale lab reactor. For a more detailed description of the system see Table 5. To calculate the energy needed to heat the substrates to the reaction temperature, literature data and models for cp-values and ΔHfus were used.| Mode of operation | Batch | Continuous | ||||
|---|---|---|---|---|---|---|
| Air stripping and water heating | Air stripping only | Evaporation and water heating | Air stripping and water heating | Air stripping only | Evaporation and water heating | |
| Height of reactor/m | 1.13 | 1.13 | 0.71 | 0.68 | 0.68 | 0.206 |
| Diameter/m | 0.34 | 0.34 | 0.43 | 0.21 | 0.21 | 0.371 |
| Height of liquid/m | 1.02 | 1.02 | 0.64 | 0.612 | 0.612 | 0.185 |
| Volume of reactor/L | 102.2 | 102.2 | 102.2 | 22.2 | 22.2 | 22.2 |
| Insulation/m | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Heat exchanger area/m2 | 4.4 | 4.0 | n/a | 5.2 | 5.2 | n/a |
| Air flow/L min−1 | 963 | 963 | n/a | 1262 | 1262 | n/a |
| Temperature of ingoing air/°C | 70 | 85 | n/a | 70 | 85 | n/a |
Conventional chemical process
Calculations for the conventional process have been made for the preheating step in the same way as for the enzymatic process using literature data for cp-values.. An estimation of the energy requirements for a conventional process has been made using the data from the calculations for the enzymatic reactor, using a reaction temperature of 150 °C.Acknowledgements
This work is a part of the research programme Greenchem at Lund University and financial support by The Foundation for Strategic Environmental Research (MISTRA) is gratefully acknowledged.Akzo Nobel Industrial Coatings AB (Malmö, Sweden) and IKEA of Sweden (Älmhult, Sweden) are gratefully acknowledged for their evaluation of the wax esters as wood coatings.
Erik Andersson, Dept. of Biotechnology, Lund University is gratefully acknowledged for the illustration in Fig. 1. The authors would also like to thank Karlshamns AB for permission to use the picture of a rapeseed field. Basti Bergdahl, Beatrice Nilsson, Kim Olofsson, Mattias Olsson and Fabian Rundbäck are gratefully acknowledged for their work on reactor modelling in the biotechnology projecting course of 2005.
References
- A. J. J. Straathof, S. Panke and A. Schmid, The production of fine chemicals by biotransformations, Curr. Opin. Biotechnol., 2002, 13(6), 548–556 CrossRef CAS
.
- S. M. Thomas, R. DiCosimo and A. Nagarajan, Biocatalysis: applications and potentials for the chemical industry, Trends Biotechnol., 2002, 20(6), 238–242 CrossRef CAS
.
- A. Schmid, Industrial biocatalysis today and tomorrow, Nature, 2001, 409(6817), 258–268 CrossRef CAS
.
- J. T. P. Derksen, F. P. Cuperus and P. Kolster, Paints and coatings from renewable resources, Ind. Crops Prod., 1995, 3(4), 225–236 CrossRef
.
- P. Anastas and J. Warner, Green Chemistry: Theory and Practice, 1998, pp. 1–160 Search PubMed
.
- P. E. Napier, et al., Enhanced organic-phase enzymatic esterification with continuous water removal in a controlled air-bleed evacuated-headspace reactor, Biotechnol. Prog., 1996, 12(1), 47–50 CrossRef CAS
.
- S. J. Kwon, et al., Removal of water produced from lipase-catalyzed esterification in organic-solvent by pervaporation, Biotechnol. Bioeng., 1995, 46(4), 393–395 CrossRef
.
- L. Gubicza, et al., Enzymatic esterification in ionic liquids integrated with pervaporation for water removal, Green Chem., 2003, 5(2), 236–239 RSC
.
- P. Mensah, J. L. Gainer and G. Carta, Adsorptive control of water in esterification with immobilized enzymes: I. Batch reactor behavior, Biotechnol. Bioeng., 1998, 60(4), 434–444 CrossRef CAS
.
- S. Bloomer, P. Adlercreutz and B. Mattiasson, Facile synthesis of fatty acid esters in high yields, Enzyme Microb. Technol., 1992, 14(7), 546–52 CrossRef CAS
.
- W. K. Teo and D. M. Ruthven, Adsorption of water from aqueous ethanol using 3-Å molecular-sieves, Ind. Eng. Chem. Process Des. Dev., 1986, 25(1), 17–21 CrossRef CAS
.
- S. Bourg-Garros, N. Razafindramboa and A. A. Paviat, Large-scale preparation of (Z)-3-hexen-1-yl acetate using candida antarctica-immobilized lipase in hexane, Biotechnol. Bioeng., 1998, 59(4), 495–500 CrossRef CAS
.
- G. Bell, et al., Methods for measurement and control of water in nonaqueous biocatalysis, Methods Biotechnol., 2001, 15(Enzymes in Nonaqueous Solvents), 105–126 Search PubMed
.
- C. M. Rosell and A. M. Vaidya, Twin-core packed-bed reactors for organic-phase enzymic esterification with water activity control, Appl. Microbiol. Biotechnol., 1995, 44(3–4), 283–6 CrossRef CAS
.
- P. J. Halling, Salt hydrates for water activity control with biocatalysts in organic media, Biotechnol. Tech., 1992, 6(3), 271–6 CAS
.
- L. Greenspan, Humidity fixed-points of binary saturated aqueous-solutions, J. Res. Nat. Bur. Stand., Sect. A, 1977, 81(1), 89–96 Search PubMed
.
- E. Wehtje, D. Costes and P. Adlercreutz, Continuous lipase-catalyzed production of wax ester using silicone tubing, J. Am. Oil Chem. Soc., 1999, 76(12), 1489–1493 CrossRef CAS
.
- C. M. Rosell, A. M. Vaidya and P. J. Halling, Continuous in situ water activity control for organic phase biocatalysis in a packed bed hollow fiber reactor, Biotechnol. Bioeng., 1996, 49(3), 284–9 CrossRef CAS
.
- E. Wehtje, et al., Water activity control in enzymic esterification processes, Enzyme Microb. Technol., 1997, 21(7), 502–510 CrossRef
.
- J. Kaur, et al., Water transfer kinetics in a water activity control system designed for biocatalysis in organic media, Enzyme Microb. Technol., 1997, 21(7), 496–501 CrossRef
.
- J. C. Jeong and S. B. Lee, Enzymic esterification reaction in organic media with continuous water stripping: effect of water content on reactor performance and enzyme agglomeration, Biotechnol. Tech., 1997, 11(12), 853–858 CrossRef CAS
.
- D. Pirozzi and G. Greco, A new experimental layout for non-aqueous enzymatic syntheses, J. Mol. Catal. B: Enzym., 2001, 11(4–6), 961–965 CrossRef CAS
.
- K. Won, J. C. Jeong and S. B. Lee, Computer-aided real-time estimation of reaction conversion for lipase-catalyzed esterification in solvent-free systems, Biotechnol. Bioeng., 2002, 79(7), 795–803 CrossRef CAS
.
- K. Won and S. B. Lee, Computer-aided control of water activity for lipase-catalyzed esterification in solvent-free systems, Biotechnol. Prog., 2001, 17(2), 258–264 CrossRef CAS
.
- T. Garcia, N. Sanchez, M. Martinez and J. Aracil, Enzymatic synthesis of fatty esters—optimisation of the synthesis of a sperm whale analogue, Enzyme Microb. Technol., 1999, 25(7), 584–590 CrossRef CAS
.
- G. Hills, Industrial use of lipases to produce fatty acid esters, Eur. J. Lipid Sci. Technol., 2003, 105(10), 601–607 CrossRef CAS
.
-
M. Martinez, Biocatalytic processes for the production of fatty acid esters, in BREW-symposium, BioPerspectives 2005, 2005, Wiesbaden, Germany Search PubMed
.
| This journal is © The Royal Society of Chemistry 2005 |
