Stability and activity of immobilised penicillin G amidase in ionic liquids at controlled aw
Alessandra
Basso
,
Sara
Cantone
,
Paolo
Linda
and
Cynthia
Ebert
*
Laboratory of Applied and Computational Biocatalysis, Dipartimento di Scienze Farmaceutiche, Università degli Studi, Piazzale Europa 1, 34127 Trieste, Italy. E-mail: ebert@units.it; Fax: +39 04052572; Tel: +39 0405583110
First published on 19th July 2005
Abstract
Despite the great interest in ionic liquids as novel solvents for biocatalysis, there is still no clear idea of their influence on the stability and the activity of enzymes. Here we analysed the activity and stability of PGA in six different ionic liquids, having different cations ([bmim] and [omim]) and anions (CH3OSO3−, PF6− and BF4−). To be active in ionic liquids, PGA-450 requires an acceptable hydration (aw > 0.60), as in organic solvents. PGA is highly stable in [bmim][PF6] and [bmim][BF4], and catalytic activity, assayed by studying the synthesis of the amide of L-phenylglycine methyl ester with methyl phenylacetate, in these ILS is comparable to that obtained in toluene.
Introduction
Twenty years have passed since the pioneering work of Klibanov on the activity of enzymes in non-aqueous media.1 It is now accepted that performing reactions in organic solvents offers undoubted advantages over aqueous media, mainly in reactions that are thermodynamically unfavoured.2,3The advantages given by the use of enzymes in organic solvents can also be achieved in ILs, and the use of room-temperature ionic liquids (RTILs) can be a good strategy to couple the advantages of biocatalysis in non-aqueous media to the increasing demand for clean technologies. The application of ionic liquids, as novel solvents or co-solvents for organic catalysis4,5 or biocatalytic transformations, has attracted considerable interest and many reviews on the subject are available.6–9 In addition, the increasing number of examples of successful applications of product extraction with supercritical fluids from ionic liquids offer the possibility to recycle both biocatalyst and IL, thus allowing the design of environmentally friendly integrated biocatalysis/separation processes.10–13
Numerous examples of biocatalysed processes in ILs presenting 1,3-dialkylimidazolium or N-alkylpyridinium cations together with a non-coordinating anion have been reported. Lipases and esterases,14–22 proteases (α-chymotrypsin, thermolysin or subtilisin),20,23–25 and recently also epoxide hydrolases,26 peroxidases,27 alcohol dehydrogenases28 or cutinases19 were demonstrated to be active in ILs, showing comparable or higher activity than in organic solvent.
Despite the growing number of examples of biocatalysed applications in ILs, the understanding and study of the influence of the properties of ILs on the enzymes has lagged behind. It is really important to develop a rational approach towards the use of ILs in order to exploit them as novel solvents also in industrial processes. Most IL properties such as polarity, hydrophobicity and solvent miscibility can be tuned through appropriate modification of the cation and anion.29
Penicillin G amidase is one of the most widely used enzymes for the industrial production of β-lactam antibiotics. In recent years, it has been demonstrated that PGA is highly active in apolar organic solvents, catalysing resolution and protection of L-amino acids and amines,30–34 and, more recently, also the protection of D-amino acids.35 This biocatalyst, which offers highly attractive opportunities in amine resolution, has a great potential in ILs, since these alternative solvents offer a highly solvating, yet non-coordinating medium in which a large number of organic and inorganic solutes can be dissolved.36
Results and discussion
In the present work we have evaluated the activity and stability of immobilised penicillin G amidase (PGA-450) in 1-alkyl-3-methylimidazolium ILs. In order to tune the properties of the ionic liquids, two different cations, 1-butyl-3-methylimidazolium and 1-octyl-3-methyl-imidazolium, were combined with inorganic (PF6− and BF4−) and organic (CH3OSO3−) anions so that six different ionic liquids were synthesised according to reported procedures (Scheme 1).20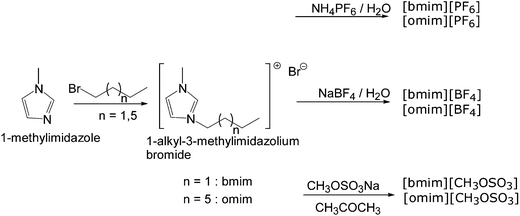 | ||
| Scheme 1 Synthesis of ILs. | ||
The characterisation by NMR spectroscopy, compared to reported data,37 confirmed the formation of the desired compounds.
After the chemical synthesis, the water content (determined by Karl Fischer titration) and the water activity (aw, measured in a sealed vessel) of the ILs were determined (see Table 1).
| Ionic liquid | Water content a (%) | a w b | k in c/h−1 | ||
|---|---|---|---|---|---|
| IL | IL/PGA-450 | IL/PGA-450 (H20, %) | |||
| a Water content was determined by Karl Fischer titration. b Water activity was measured using a hygrometer (DARAI, Trieste) after 24 h equilibration. Conditions: T = 30 °C, 1 mL of pure IL, with the addition of 100 mg of wet PGA-450 (containing 35 µL water) and additional water, which is indicated in parentheses. c Inactivation constants were determined by suspending the enzymatic preparation in pure IL and measuring the residual activity through the NIPAB assay. | |||||
| [bmim][PF6] | 1.5 | 0.30 | 0.62 | 0.66 (1) | <10−5 |
| [omim][PF6] | 0.3 | 0.30 | 0.76 | 0.80 (1) | 0.35 |
| [bmim][BF4] | 0.3 | 0.17 | 0.40 | 0.70 (10) | <10−5 |
| [omim][BF4] | 2.0 | 0.21 | 0.53 | 0.76 (5) | 0.0065 |
| [bmim][CH3OSO3] | 11.4 | 0.19 | 0.40 | 0.71 (20) | 0.13 |
| [omim][CH3OSO3] | 7.3 | 0.20 | 0.44 | 0.78 (20) | 0.41 |
As previously reported, the anion greatly influences the water miscibility and the maximum amount of retained water. Literature data show that both [Cnmim][PF6] and [omim][BF4] are only partially water miscible (see footnote† for details about water miscibility),29,38,39 while [Cnmim][CH3OSO3] and [bmim][BF4] are all water soluble.
However, for any given solvent, there will be a characteristic relationship between water activity (aw) and water concentration. Water activity depends on the specific interactions between solvent and water molecules, and in solvents that solubilize water these interactions are favorable, resulting in low values of aw. In poor solvents, at a given molar fraction of water, aw is much larger.40
ILs with the more hydrophobic [PF6] as anion, have the higher aw (0.30), while tetrafluoroborates and methylsulfates show lower and similar aw, despite differences in the water content.
It is widely reported that the activity of enzymes as amidases, glycosidases and proteases in non-aqueous media, organic solvents or ionic liquids, strongly depends on the water activity (aw) of the system.
We have previously demonstrated that, in dichloromethane or toluene, immobilised PGA is active at aw above 0.5, showing a maximum of activity at a value of about 0.8.31
From data in Table 1 it clearly appears that the ionic liquids, as obtained, could not be directly used for PGA biocatalysis, since they are not sufficiently hydrated (in all cases aw is lower than 0.3).
The enzymatic preparation used in the present study is a covalently immobilised form of penicillin G amidase (PGA-450) that is supplied in a wet form (it contains 35% water). Thus, when PGA-450 was suspended in the ionic liquids, the aw of the overall system increased. Since the hydrophobic character of the ionic liquid is mainly determined by the anion,29 PGA-450 gives the highest values of aw (0.62 and 0.76) when suspended in [bmim][PF6] and [omim][PF6]. The cation influences the aw of the system, but to a minor extent. ILs with [omim] as the cation give, as expected, higher values of aw if compared to the analogous ILs with [bmim].
As a consequence, we added the necessary amount of water to the systems formed by PGA-450 in ILs in order to obtain aw compatible with enzyme activity: in [bmim][PF6] and [omim][PF6] the addition of 1% water was sufficient, whereas for the more hydrophilic ILs, [bmim][BF4] and [omim][BF4], the addition of 10% and 5% water, respectively, was necessary to obtain aw of 0.70–0.76. Both [Cnmim][CH3OSO3] required the addition of 20% water to obtain values of aw compatible with enzyme activity (Fig. 1). Our data are in agreement with results obtained by Kragl and co-workers, who reported that ILs with an alkyl sulfate as the anion required percentages of water of up to 50% to obtain a water activity of about 0.8.41,42
![Variation, upon addition of water, of the aw of the system formed by PGA-450 suspended in [bmim][CH3OSO3]
(empty squares) and [omim][CH3OSO3]
(black squares). Conditions: 1 mL IL, 100 mg PGA-450, system equilibrated for 24 h at 30 °C.](/image/article/2005/GC/b506230f/b506230f-f1.gif) | ||
| Fig. 1 Variation, upon addition of water, of the aw of the system formed by PGA-450 suspended in [bmim][CH3OSO3] (empty squares) and [omim][CH3OSO3] (black squares). Conditions: 1 mL IL, 100 mg PGA-450, system equilibrated for 24 h at 30 °C. | ||
Stability of PGA in ILs
The effect of ILs on the stability of immobilised PGA was assayed by suspending the enzyme in ILs, and determining the activity over time. After being suspended in ILs, the enzyme was washed with buffer and the residual activity was determined by the standard NIPAB assay.43PGA-450 is a highly stable preparation and, when suspended in toluene or buffer, it fully maintains its original activity for up to several weeks. Results reported in Table 1 show that PGA-450 suspended in [bmim][CH3OSO3] or [omim][CH3OSO3] completely lost its activity in a few hours (see Fig. 2). The stability of the biocatalyst was also assayed in the presence of additional water (20% added water) and the inactivation constants were found comparable. The inactivation of the biocatalyst in these ILs presumably depends on unfavourable interactions of methyl sulfate anion with PGA. Detrimental effects of alkyl sulfates are already reported in the literature.21,41,42
![Stability (activity over time) of PGA-450 suspended in [bmim][CH3OSO3]
(black circles) and [omim][CH3OSO3]
(empty circles).](/image/article/2005/GC/b506230f/b506230f-f2.gif) | ||
| Fig. 2 Stability (activity over time) of PGA-450 suspended in [bmim][CH3OSO3] (black circles) and [omim][CH3OSO3] (empty circles). | ||
The cation exerts a strong effect on the denaturation of PGA-450, since the enzyme is more stable when suspended in the ILs with [bmim] as cation. PGA suspended in [bmim][PF6] or [bmim][BF4], maintained its activity after one week of exposure, showing a stability similar to that observed in organic solvent (toluene) or Kpi buffer. The [omim] cation causes a gradual loss in activity of the enzyme suspended in [omim][PF6] or [omim][BF4] (Fig. 3). This effect was more evident in the more hydrophobic [omim][PF6], with an inactivation constant that was almost fifty times higher than the kin observed for [omim][BF4].
![Stability (activity over time) of PGA-450 suspended in [omim][BF4]
(black squares) and [omim][PF6]
(empty squares).](/image/article/2005/GC/b506230f/b506230f-f3.gif) | ||
| Fig. 3 Stability (activity over time) of PGA-450 suspended in [omim][BF4] (black squares) and [omim][PF6] (empty squares). | ||
In contrast to the behaviour in organic solvents, where the enzyme stability increases at high values of logP, the stability in ILs seems to be due to a fine balance between the hydrophobicity and the hydrophilicity of the cation and anion.
Recently it has been suggested that the enzymatic activity of lipase CALB in ionic liquids results from a fine balance of hydrogen bond-accepting and donating properties, that maintain structural integrity of the α-helices and β-sheets and prevent the protein unfolding.22 Furthermore, other factors, such as free volume contributions, ionic interactions and confinement effects, may also contribute to protein stabilization,43 and spectroscopic studies of stability of α-chymotrypsin confirm that the enzyme stabilization is associated with structural changes of the protein.24 These studies regard the enzyme in the native form, while PGA-450 is immobilized. We are currently investigating the possibility of applying alternative techniques suitable for studying insoluble enzymes.
Catalytic activity of PGA in ILs
The catalytic activity of PGA-450 was assayed in ILs by studying the synthesis of the amide of L-phenylglycine methyl ester with methyl phenylacetate working at equimolar concentrations of reagents (Scheme 2).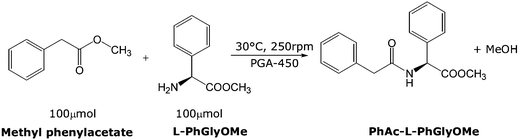 | ||
| Scheme 2 PGA catalysed synthesis of PhAc-L-PhGlyOMe in ILs and toluene. | ||
The results are reported in Table 2, together with the data obtained in toluene. In order to perform the reactions at comparable aw values, water was added to the ILs as shown in Table 1. No addition of water was necessary to PGA-450 suspended in toluene (the ratio of biocatalyst in solvent was maintained at 10% w/v), since, in these conditions, the water retained by the enzymatic preparation is sufficient to guarantee optimal aw for synthetic PGA activity (aw ∼ 0.8).31 The synthesis of the amide in [bmim][PF6] and [bmim][BF4]—where PGA-450 is fully stable—was efficient as in toluene (complete synthesis without any side reaction or hydrolysis of reagents or product), using the same amount of catalytic units. The reactions carried out in toluene and [bmim][PF6] were complete in three hours, whereas reactions performed in [bmim][BF4] were complete after 24 hours.
| Solvent | Viscosity (cP, 30 °C) | Initial rate/µmol min−1 |
|---|---|---|
| Conditions: 1mL solvent, 100 µmol of PhAcOMe and L-PhGlyOMe, 30 °C in blood rotator. Before the addition of reagents 1% and 10% water was added to [bmim][PF6] and [bmim][BF4] respectively. | ||
| Toluene | 0.59045 | 1.244 |
| [bmim][PF6] | 20429 | 1.027 |
| [bmim][BF4] | 9129 | 0.813 |
From results reported in Table 2, it is remarkable to observe that, despite the high viscosity of both ILs that critically affects mass transfer, the initial rates determined in [bmim][PF6] and [bmim][BF4] were similar to that measured in toluene.
The synthetic activity was also assayed in the other ionic liquids. Figs. 4 and 5 show the yields achievable in [omim][PF6] and [omim][BF4] at different aw values. At high values of aw the initial rate of the reaction increases, and prevails on the inactivation effect of the ILs.
![Synthesis of PhAc-l-PhGlyOMe in [omim][PF6]. Conditions: T
= 30 °C, 1 mL IL, 100 mg PGA-450, 0 µL H2O, aw
= 0.76 (black circles), 10 µL H2O, aw
= 0.80 (black squares).](/image/article/2005/GC/b506230f/b506230f-f4.gif) | ||
| Fig. 4 Synthesis of PhAc-L-PhGlyOMe in [omim][PF6]. Conditions: T = 30 °C, 1 mL IL, 100 mg PGA-450, 0 µL H2O, aw = 0.76 (black circles), 10 µL H2O, aw = 0.80 (black squares). | ||
![Synthesis of PhAc-l-PhGlyOMe in [omim][BF4]. Conditions: T
= 30 °C, 1 mL IL, 100 mg PGA-450, 0 µL H2O, aw
= 0.53 (black squares), 30 µL H2O, aw
= 0.70 (empty triangles), 50 µL H2O, aw
= 0.76 (black circles).](/image/article/2005/GC/b506230f/b506230f-f5.gif) | ||
| Fig. 5 Synthesis of PhAc-L-PhGlyOMe in [omim][BF4]. Conditions: T = 30 °C, 1 mL IL, 100 mg PGA-450, 0 µL H2O, aw = 0.53 (black squares), 30 µL H2O, aw = 0.70 (empty triangles), 50 µL H2O, aw = 0.76 (black circles). | ||
The inactivation rate of PGA-450 caused by [omim][BF4] is lower than the rate observed for [omim][PF6], so that, at aw = 0.76, complete conversion can be achieved thanks to favourable kinetics. Nevertheless, a small decrease in the aw of the system reduces the synthetic rate in [omim][BF4], causing the reaction to stop at 80% (aw = 0.70); at aw = 0.53 the yield is only 20%. In [omim][PF6], at aw = 0.76 or 0.80, yields of 15% or 60% were obtained respectively.
[bmim][CH3OSO3] and [omim][CH3OSO3] require an addition of 20% water to reach optimal aw values, thus obtaining systems that are more similar to co-solvent aqueous mixtures. PGA-450 is highly stable in apolar solvents,31 but, as with many other enzymes, it suffers the presence of polar co-solvents. When suspended in ILs–water mixtures the activity of PGA-450 decreased rapidly and no synthetic activity was observed.
Conclusions
Here we report on a study of activity and stability of immobilised penicillin G amidase (PGA-450) in different ionic liquids. The study was conducted in ionic liquids with 1-alkyl-3-methylimidazolium cations, looking at the effect of both the anion and the alkyl chain of the cation on the activity, and most importantly, on the stability of the enzyme. The stronger effect on the stability of the biocatalyst is given by the anion. PGA is fully stable in [bmim][PF6] and [bmim][BF4] and shows good synthetic activity in these ionic liquids. The initial rates of synthetic reactions performed in these ILs and in toluene are of the same order of magnitude. The reactions were performed in ILs at equimolar concentrations of reactants, without any observed side hydrolysis.Similarly to its behaviour in hydrophobic organic solvents, PGA in ILs requires an optimal hydration (aw of about 0.80). This value was obtained by adding between 1% and 20% water, depending on the nature of the ILs.
ILs with methylsulfate as the anion require more than 20% added water to achieve an aw value compatible with enzyme activity. However, even at optimal hydration of the enzyme, no synthetic activity was observed due to the strong denaturising effect of such ILs.
In conclusion, despite the high viscosity of these media, the synthesis catalysed by PGA is highly efficient in ionic liquids, thus achieving a promising basis for the development of environmentally benign methodology. We believe that a rational screening among ILs could lead to their successful application in the resolution of racemic compounds, in particular for the production of enantiomerically pure or enriched amines.
Experimental
Materials
All chemicals, 1-methylimidazole, 1-bromobutane, 1-bromooctane, ammonium hexafluorophosphate, sodium tetrafluoroborate, sodium methylsulfate, 5-amino-2-nitrobenzoic acid (NABA), cinnamyl alcohol, methyl phenylacetate (PhAcOMe), L-phenylglycine methyl ester hydrochloride (L-PhGlyOMe·HCl) and phenylacetyl chloride were obtained from Sigma-Aldrich–Fluka or Lancaster and were used without further purification.Toluene (RUDI PONT) used in enzymatic reactions was previously dried over molecular sieves (4 Å). Ethyl acetate and acetone were from Sigma-Aldrich. Ultrapure water (18.2 Ω cm−1) was obtained with MilliQ Plus (Millipore) and was used for HPLC mobile phases preparation and in the enzymatic reactions.
PGA-450 (35% water content), kindly donated by Roche, consists of penicillin G amidase from Escherichia coli covalently immobilised on a polymer, the chemical structure of which is not disclosed by the supplier.
Synthesis of NIPAB [2-nitro-5-(phenylacetylamino) benzoic acid]
5-Amino-2-nitrobenzoic acid (NABA, 0.05 mol, 9.1 g) was combined in a flask with 2,6-lutidine (0.1 mol, 10.7 g) in CH2Cl2 and maintained in an ice bath. Phenylacetyl chloride (0.075 mol, 22.5 g) was added dropwise and the mixture was maintained under stirring overnight. The organic phase was washed with HCl 0.1 M, NaOH 0.1 N and H2O, then the organic solvent was dried on MgSO4 and removed under reduced pressure. 6.37 g of brown crystals were obtained (70% yield). NIPAB was characterised by 1H NMR and 13C NMR and data compared to literature.44Preparation of free L-PhGlyOMe from the corresponding hydrochloride salt
Equimolar amounts (0.005 mol) of L-PhGlyOMe·HCl and NaHCO3·10H2O were suspended in CH2Cl2 and the mixture was stirred for 30 min at 30 °C, then filtered. CH2Cl2 was removed under reduced pressure. 0.7 g of yellow oil was obtained (85% yield). L-PhGlyOMe was characterised by 1H NMR and 13C NMR and data compared to literature.34Water content
The water content of each preparation was determined by Karl Fischer titrations.Water activity
The water activity aw of ILs, of ILs with PGA-450 and of ILs with PGA-450 and added water (Table 1) was measured using a hygrometer Darai (Trieste, Italy). Measurements were carried out by sealing the sensor into the open end of 5 mL glasses vials, thermostatted at 30 °C, until constant reading. All samples were previously equilibrated for 24 h.Enzyme stability in ionic liquids
20 mg of PGA-450 were added to 1 mL of ILs. Samples were mixed in an orbital shaker at 25 °C and 180 rpm, then filtered (0.45 µm) and washed with Kpi buffer (KH2PO4, 0.02 M, pH 8.0).2 mL Kpi buffer and 1 mL of NIPAB solution (0.015 M in KH2PO4) were added to the enzyme and samples were mixed for 10 minutes at 180 rpm in an orbital shaker (Scheme 3).
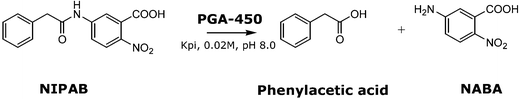 | ||
| Scheme 3 Activity assay of PGA by hydrolysis of the chromogenic NIPAB substrate to phenylacetic acid and NABA. | ||
Samples were then filtered (0.45 µm) and analysed with a UV-spectrophotometer (Perkin Elmer Lambda 20). The conversion of NIPAB to NABA was detected at 405 nm (ε405 = 9090). One unit of activity was defined as the amount of enzyme required to produce 1 µmol of NABA at 25 °C.
Synthetic activity in ionic liquids
PGA-450 (100 mg), ionic liquid (1 mL) and water (where indicated) were mixed in a 5 mL glass vial. Reactions were started upon addition of 100 µmol of both L-PheGlyOMe (16.5 µL) and PhAcOMe (14.4 µL). Reactions were incubated in a thermostatted orbital shaker, at 30 °C.Samples of 100 µL from the reaction were dissolved in 900 µL of MeCN and were analysed by HPLC (HPLC Gilson-351 equipped with a C-18 Supelco column, flow 1 mL min−1, elution isocratic: MeCN ∶ H2O = 40 ∶ 60, 0.02% trifluoroacetic acid).
The formation of the product was evaluated by using an internal standard (cinnamyl alcohol).
Acknowledgements
Thanks are due to M.I.U.R. (Rome, Italy) for financial support to P. L. We thank Prof. L. Gardossi for fruitful discussions.References
- A. Zaks and A. M. Klibanov, Science, 1986, 224, 1249.
- A. M. Klibanov, Nature, 2001, 409, 241 CrossRef CAS.
- A. M. Klibanov, CHEMTECH, 1986, 16, 354 CAS.
- R. Sheldon, Chem. Commun., 2001, 2399 RSC.
- J. D. Holbrey and K. R. Seddon, Clean Prod. Process., 1999, 1, 223 CrossRef.
- P. Seongsoon and R. J. Kazlauskas, Curr. Opin. Biotechnol., 2003, 14, 432 CrossRef CAS.
- U. Kragl, M. Eckstein and N. Kaftzik, Curr. Opin. Biotechnol., 2002, 13, 565 CrossRef CAS.
- (a) F. van Rantwijk, R. Madeira Lau and R. A. Sheldon, TIBTECH., 2003, 3, 131 Search PubMed; (b) Z. Yang and W. Pan, Enzyme Microb. Technol., 2005, 37, 19 CrossRef CAS.
- C. F. Poole, J. Chromatogr. A, 2004, 1037, 49 CrossRef CAS.
- (a) M. T. Reetz, W. Wiesenhöfer, G. Franciò and W. Leitner, Chem. Commun., 2002, 992 RSC; (b) M. T. Reetz, W. Wiesenhöfer, G. Franciò and W. Leitner, Adv. Synth. Catal., 2003, 345, 1221 CrossRef CAS.
- S. V. Dzyuba and R. A. Bartsch, Angew. Chem., Int. Ed., 2003, 42, 148 CrossRef CAS.
- P. Lozano, T. De Diego, S. Gmouh, M. Vaultier and J. L. Iborra, Biotechnol. Prog., 2004, 20, 661 CrossRef CAS.
- S. Garcia, N. M. T. Lourenço, D. Lousa, A. F. Sequiera, P. Mimoso, J. M. S. Cabral, C. A. M. Afonso and S. Barreiros, Green Chem., 2004, 6, 466 RSC.
- M. Persson and U. T. Bornscheuer, J. Mol. Catal. B: Enzym., 2003, 22, 21 CrossRef CAS.
- A. Kamal and G. Chouhan, Tetrahedron Lett., 2004, 45, 8801 CrossRef CAS.
- M. Noël, P. Lozano, M. Vaultier and J. L. Iborra, Biotechnol. Lett., 2004, 26, 301 CrossRef CAS.
- T. Itoh, Y. Nishimura, N. Ouchi and S. Hayase, J. Mol. Catal. B: Enzym., 2003, 26, 41 CrossRef CAS.
- R. Irimescu and K. Kato, J. Mol. Catal. B: Enzym., 2004, 30, 189 CrossRef CAS.
- S. J. Nara, J. R. Harjani and M. M. Salunkhe, Tetrahedron Lett., 2002, 43, 2979 CrossRef CAS.
- (a) M. J. Kim, H. M. Kim, D. Kim, Y. Ahn and J. Park, Green Chem., 2004, 6, 471 RSC; (b) J. A. Laszlo and D. L. Compton, Biotechnol. Bioeng., 2001, 75, 181 CrossRef CAS.
- P. Lozano, T. De Diego, D. Carrié, M. Vaultier and J. L. Iborra, J Mol. Catal. A: Chem., 2004, 214, 113 CrossRef CAS.
- R. Madeira Lau, M. J. Sorgedrager, G. Carrea, F. van Rantwijk, F. Secundo and R. A. Sheldon, Green Chem., 2004, 6, 483 RSC.
- M. Erbendilger, A. J. Mesiano and A. J. Russel, Biotechnol. Prog., 2000, 16, 1129 CrossRef CAS.
- P. Lozano, T. De Diego, D. Carriè, M. Vaultier and J. L. Iborra, J. Mol. Catal. B: Enzym., 2003, 21, 9 CrossRef CAS.
- M. Eckstein, M. Sesing, U. Kragl and P. Adlercreutz, Biotechnol. Lett., 2002, 24, 867 CrossRef CAS.
- C. Chiappe, E. Leandri, S. Lucchesi, D. Pieraccini, B. D. Hammock and C. Morisseau, J. Mol. Catal. B: Enzym., 2004, 27, 243 CrossRef CAS.
- K. Okrasa, E. Guibé-Jampel and M. Therisord, Tetrahedron: Asymmetry, 2003, 14, 2487 CrossRef CAS.
- M. Eckstein, M. V. Filho, A. Liese and U. Kragl, Chem. Commun., 2004, 1084 RSC.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156 RSC.
- D. Roche, K. Prasad and O. Repic, Tetrahedron Lett., 1999, 40, 3665 CrossRef CAS.
- A. Basso, L. De Martin, C. Ebert, L. Gardossi, P. Linda and V. Zlatev, J. Mol. Catal. B: Enzym., 2001, 11, 851 CrossRef CAS.
- A. Basso, S. Biffi, L. De Martin, L. Gardossi and P. Linda, Croat. Chim. Acta, 2001, 74, 757 CAS.
- A. Basso, P. Braiuca, L. Gardossi, C. Ebert, P. Linda and F. Benedetti, Biochim. Biophys. Acta, 2002, 1601, 85 CAS.
- A. Basso, P. Braiuca, L. De Martin, C. Ebert, L. Gardossi and P. Linda, Tetrahedron: Asymmetry, 2000, 11, 1789 CrossRef CAS.
- C. Carboni, P. J. L. M. Quaedflieg, Q. B. Broxterman, P. Linda and L. Gardossi, Tetrahedron Lett., 2004, 45, 9649 CrossRef CAS.
- L. A. Blanchard and J. F. Brennecke, Ind. Eng. Chem. Res., 2001, 40, 287 CrossRef CAS.
- S. T. Lin, M. F. Ding, C. W. Chang and S. S. Lue, Tetrahedron, 2004, 60, 9441 CrossRef CAS.
- S. Rivera-Rubero and S. Baldelli, J. Am. Chem. Soc., 2004, 126, 11788 CrossRef CAS.
- M. Koel, Proc. Est. Acad. Sci. Chem., 2000, 49, 145 Search PubMed.
- G. Bell, A. E. M. Janssen and P. J. Halling, Enzyme Microb. Technol., 1997, 20, 471 CrossRef CAS.
- N. Kaftzik, P. Wasserscheid and U. Kragl, Org. Proc. Res. Dev., 2002, 6, 553 Search PubMed.
- N. Kaftzik, W. Kroutil, K. Faber and U. Kragl, J. Mol. Catal. A: Chem., 2004, 214, 107 CrossRef CAS.
- S. N. Baker, M. McCleskey, S. Pandey and G. A. Baker, Chem. Commun., 2004, 940 RSC.
- W. B. L. Alkema, R. Floris and D. B. Janssen, Anal. Biochem., 1999, 275, 47 CrossRef CAS.
- Handbook of Chemistry and Physics, ed. R. C. Weast, CRC Press Inc., Cleveland, Ohio, 57th edn., 1977, p. F-57 Search PubMed.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC.
Footnote |
| † Water is only partially miscible in [bmim][PF6] and [omim][PF6]: 11700 and 6666 ppm respectively.29 Holbrey and Seddon reported that for [Cnmim][BF4], those with n < 6 are water soluble, although to varying degrees: water is partially miscible in [omim][BF4]: 110000 ppm.46 |
| This journal is © The Royal Society of Chemistry 2005 |
