Progress in evaluation of risk potential of ionic liquids—basis for an eco-design of sustainable products†
Bernd
Jastorff
a,
Kerstin
Mölter
a,
Peter
Behrend
a,
Ulrike
Bottin-Weber
a,
Juliane
Filser
a,
Anna
Heimers
a,
Bernd
Ondruschka
c,
Johannes
Ranke
*a,
Maike
Schaefer
a,
Heike
Schröder
a,
Annegret
Stark
c,
Piotr
Stepnowski
b,
Frauke
Stock
a,
Reinhold
Störmann
a,
Stefan
Stolte
a,
Urs
Welz-Biermann
d,
Susanne
Ziegert
a and
Jorg
Thöming
a
aUFT - Centre for Environmental Research and Technology, University of Bremen, Leobener Straße, D-28359 Bremen, Germany. E-mail: jranke@uni-bremen.de
bFaculty of Chemistry, University of Gdansk, Ul. Sobieskiego 18/19, PL-80-952 Gdańsk, Poland
cInstitute for Technical and Environmental Chemistry, Friedrich-Schiller-University Jena, Lessingstraße 12, D-07743 Jena, Germany
dMerck KGaA, New Ventures Materials, Frankfurter Straße 250, D-64293 Darmstadt, Germany
First published on 19th April 2005
Abstract
Motivated by the prevailing need for a sustainable development and taking the principles of Green Chemistry as a starting point, the present paper describes new and updated findings regarding a sustainable product design for ionic liquids. The focus is on environmental risk. Nevertheless, cytotoxicity testing and first indicative results from a genotoxicity study extend present knowledge also with regard to possible effects on humans. The structural variability of commercially available ionic liquids as well as the abundance of theoretically accessible ionic liquids is illustrated and the consequences for an integrated risk assessment accompanying the development process are discussed. The side chain effect on toxicity for imidazolium type ionic liquids was confounded by more complex biological testing. Also, an influence of an anion on cytotoxicity is shown for the first time. Testing of presumed metabolites of the imidazolium type cations showed a significantly lower biological activity in cytotoxicity studies than their parent compounds. The importance of a purity assessment for ionic liquids is pointed out and a collection of methods that is believed to be adequate is presented. In addition to risk analysis, the use of life cycle analysis for the multi-objective problem of designing ionic liquids is sketched and an eco-design scheme for ionic liquids is proposed. In conclusion, the paper illustrates the complex nature of the development processes ionic liquids are currently undergoing and provides guidance on which aspects have to be kept in mind.
 Project Team Ionic Liquids | The interdisciplinary “Project Team Ionic Liquids” at the Centre for Environmental Research and Technology (UFT) in Bremen is coordinated by Prof. Bernd Jastorff and was founded in May 2002. In several projects, technicians, PhD students, post-docs and professors from different departments (Bioorganic Chemistry, Process Integrated Waste Minimisation, General and Theoretical Ecology, Epidemiology) are working together combining the specialised knowledge of their different disciplines. Important national and international network partners are the Pomeranian Centre for Environmental Research and Technology (POMCERT) in Gdańsk (Poland) and the Merck KGaA in Darmstadt (Germany). |
Introduction
Chemistry plays a very important role in Sustainable Development as has been pointed out in chapter 19 of Agenda 21.1 For today's chemists, designing benign industrial chemicals, products and processes is both a vision and a mission.The principles of Green Chemistry as proposed by Anastas and Warner2 in 1998 offer guidelines as to how chemists can direct their efforts towards more sustainability in research and development of new chemical entities and products. Principle 4 and 10 direct their design strategy.
Chemical products should be designed to preserve efficacy of function while reducing toxicity.
(4th Principle of Green Chemistry2)
Chemical products should be designed so that at the end of their function they do not persist in the environment, and break down into innocuous degradation products.
(10th Principle of Green Chemistry2)
To fulfil these missions, chemists have to network their synthetic efforts with other disciplines, because designing safer chemicals is an inter-/transdisciplinary challenge. In development of new drugs or pesticides this collaboration is the state of the art in industry. Due to economic and traditional reasons this is currently not standard practice in the development of new industrial chemicals. Is it possible to make economic use of the design strategy and the tremendous experience gained in risk assessment of chemicals and their degradation products in pharmaceutical and pesticide development without concurrently raising the development costs to the same dimensions as in pharmaceutical industry? We are far from being able to provide an adequate answer to this question at this moment, but our transdisciplinary team is evaluating the chances offered by a work-sharing cooperation between academia, industry and small and medium enterprises (SMEs).
Ionic liquids—a model for a sustainable design of new chemical entities and products
Our model case for the development of sustainable industrial chemicals is the fascinating class of new organic solvents called “ionic liquids” (ILs). As has been pointed out by Seddon,3 Wasserscheid and Welton,4 Rogers and Seddon5 and others, ILs have the potential to improve existing processes and new developments in a multitude of fields in chemistry and chemical technology.3–5 Beside their advantages in chemical synthesis, catalysis, extraction, biotechnology, electrochemistry and other applications, they offer one very important advantage compared to classical volatile organic solvents: They possess no detectable vapour pressure and thus—provided that they are both pure and stable—do not emit volatile organic compounds. Occupational health in and technical safety of new processes are strongly improved. But does this already make them “green” or even “sustainable” chemicals?In 2003 we discussed this question and offered a transdisciplinary strategy to assess potential risks of ionic liquid entities and design sustainable products.6 In short, we proposed the following tools:
• interdisciplinary theoretical and work-sharing experimental collaboration
• selection of lead chemicals according to the “testkit concept”
• (eco)toxicological test battery on different levels of complexity
• assessment of the molecular interaction potential, shape and conformational flexibility, chemical and biochemical reactivity of a chemical entity from a systematic algorithm
• evaluation of qualitative and quantitative structure–activity relations (T-SAR7,8/QSAR)
• theoretical assessment of presumable transformation products due to metabolic reactions based on T-SAR
• multidimensional risk analysis (release, spatiotemporal range, bioaccumulation, biological activity and uncertainty)9
This strategy is directed at the design of products with high process efficiency, acceptable costs and low toxic potential for man and the environment.
We are aware that these objectives are idealistic ones, which will often produce dilemmas and conflicts of objectives. But one should keep in mind that the main paradigm change in thinking and acting introduced by Agenda 21 is indeed not to fight for either economical, social or ecological needs, but to commonly search for an acceptable compromise. Successful pursuance of this aim requires the development of close partnerships between different R&D institutions and industrial stakeholders within the areas of production and application to allow for a fruitful discussion about design parameters and product profiles.
Fig. 1 shows the interrelations between the objectives of sustainable product design and the aspects of structural elements of chemicals to be optimised within a design process.6
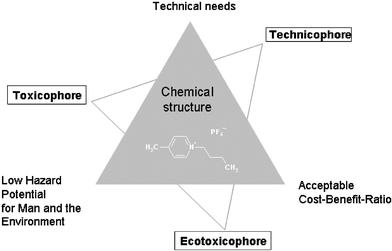 | ||
| Fig. 1 Aspects of sustainable product design. | ||
Collaboration between academia and industry
Green chemicals may be designed in academia alone, but development of sustainable industrial products and processes need the experience and input of chemical industry. Therefore our academic team and Merck KGaA, New Business Chemicals, Darmstadt, Germany, joined into a strategic partnership. The work-shared objectives are i) assessing (eco)toxicological hazards of ionic liquid entities, ii) developing a toolbox of structural elements of ionic liquids which can be used in order to reduce the hazard potential for man and the environment, (iii) fine tuning of chemical structures of ionic liquids tailormade for defined technological applications at an acceptable cost–benefit ratio. Lead chemicals to assess the (eco)toxicological data as defined according to the T-SAR guided testkit concept6 will be synthesized by the industrial partner, risk relevant data will be measured by the UFT team and the industrial partner depending on the special expertise existing in either institution. While technological needs will be defined by customers of Merck KGaA, technical solutions will be developed in a joint effort.Compared to the suggestions published earlier6 by the UFT team this paper presents an improved strategy to consider the whole life cycle of a new chemical at the earliest level of development. The algorithm for a sustainable product design is orientated to the needs of special applications. It includes the assessment of the purity of ionic liquids used in technical applications (“technical grade”) and in biological testing (“analytical grade”). Therefore we will briefly present a set of analytical methods necessary to discuss the question of purity of ionic liquid entities.
We will also present new (eco)toxicological data demonstrating whether and how structural elements influence toxicity at different levels of biological complexity. The biological data given here are only representative and exemplary to support our conclusions with respect to design criteria. Experimental details of biological testing and the full range of data obtained will be published elsewhere.
The pool of ionic liquid entities—dilemma or chance?
Ionic liquids are a class of new chemical compounds that are the target of rapidly growing scientific and technological interest, indicated by a continuously growing number of publications and new chemical entities. The first successful implementation of an industrial process has been recently reported.10Simply claiming that ionic liquids are the solvents of the future would mean hiding a dilemma. In fact, the term ionic liquids comprises an indefinite number of diverse chemical entities, so far 1012 accessible compounds have been estimated.11 Also indefinite is the number of possible technological applications and usage patterns of ionic liquids, their individual life cycles (including recycling and waste treatment) and, last but not least, the targets for biological effects on man and the environment at all levels of biological complexity.
This dilemma excludes a screening process or the “let's try” approach to find a specific ionic liquid for a specific technology or use that fulfils the criteria to be a sustainable chemical product or process.
On the other hand the huge number of designable chemical entities opens up the chance of tuning the structural elements of an ionic liquid in a way that the principles of Green Chemistry and sustainable development can be fulfilled in an acceptable manner. As pointed out already this includes the discussion and solution of conflicts between technological needs, (eco)toxicological acceptance and economical chances. It is the chemist's opportunity to guide such development processes by designing and synthesising tailored ionic liquid entities within a multidisciplinary network. This process has already started, in a somehow systematic manner.
To define the structural elements of a toolbox aimed at supporting the design of sustainable ionic liquids, we analysed the structures of existing ionic liquids according to T-SAR. We defined three types of substructures: the positively charged moiety we call “headgroup”, the one, two or more side chains R1, R2etc. which represent substituents on this headgroup and the type of anion. Figs. 2 and 3 give a selection of those substructures already for sale. Through collaboration, our network has access to more than 140 different ionic liquid entities at the moment.
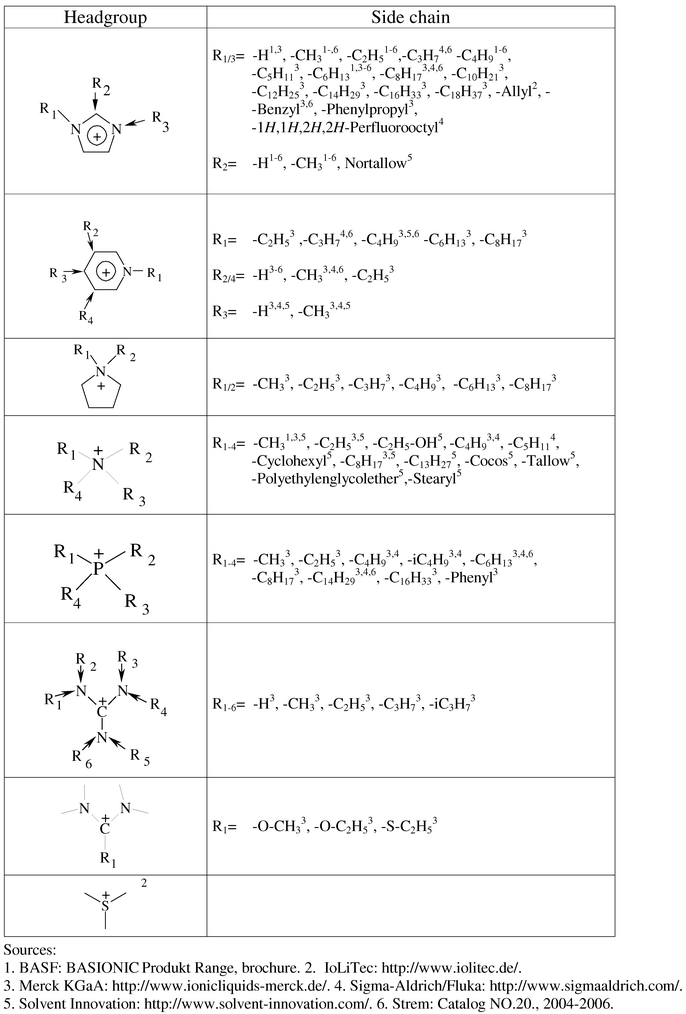 | ||
| Fig. 2 Selection of headgroups and side chains used in commercially available ionic liquids. This list is not meant to be complete. | ||
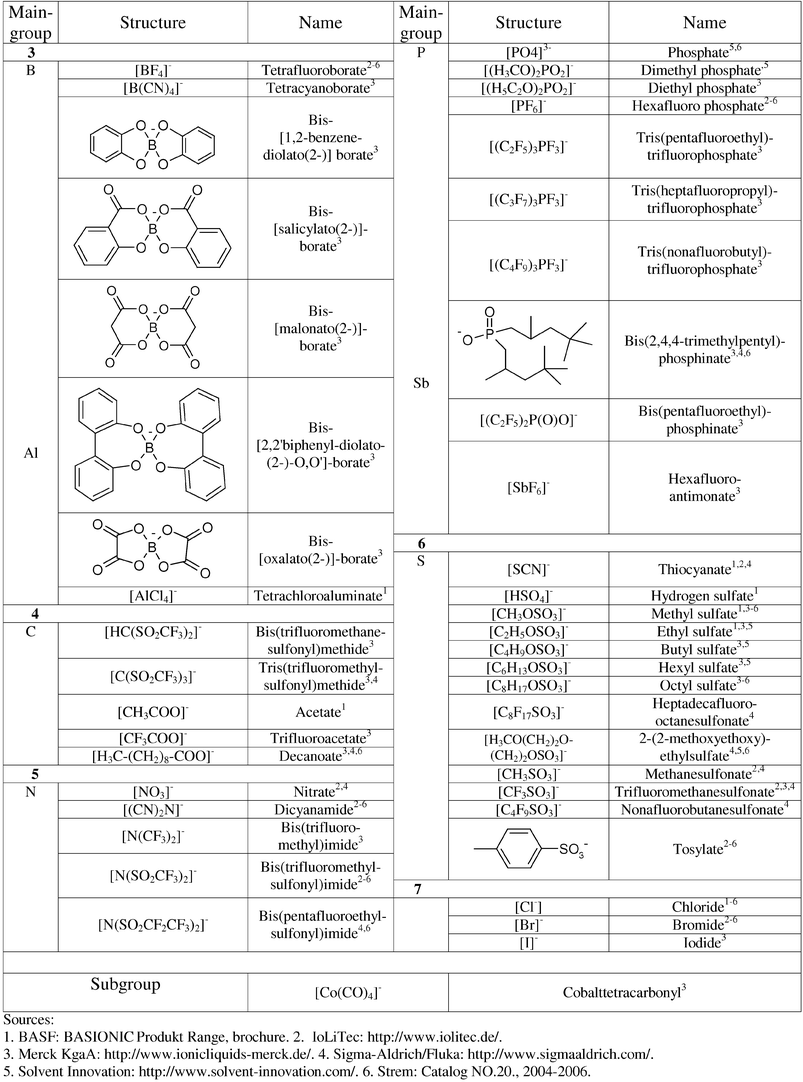 | ||
| Fig. 3 Selection of anions currently used in commercially available ionic liquids. This list is not meant to be complete. | ||
With increasing knowledge of the biological effects that different structural elements have on the (eco)toxicity of ionic liquids (“toxicophore” and “ecotoxicophore”, cf.Fig. 1) as well as their intrinsic influence on physical and chemical properties which determine the usage pattern of ionic liquids (“technicophore”) the design process will overcome the synthesis of new ionic liquids merely directed by chemical interest. Thus the pool of theoretically existing ionic liquid chemical entities will be used rationally and not by chance.
A flexible (eco)toxicological test battery
The classical hazard assessment of new industrial chemicals is directed by regulations, standardised procedures and is thus inflexible. The number and complexity of biological test systems and thus the costs of the evaluation increase with the proposed production volume of a chemical.From an economical point of view it is impossible to perform such expensive procedures for all chemicals and processes in the development process, especially as many chemical entities are only potential candidates.
Therefore we advocate the concept of a flexible test strategy for ionic liquids and other industrial chemicals (e.g. antifouling biocides)12,13 during their design and development phase. For the selection of test systems, considerations about the information value and the cost–benefit ratio of test results are taken into account. The number of chemical entities (“lead chemicals”) tested in a distinct biological system is matched to the input of money and time needed for a specific test.
While test systems at higher levels of complexity allow for a better understanding of the effects of ionic liquids on a complex environment, molecular and cellular test systems are useful to improve our knowledge about the impact of specific structural elements and the way they should be modified in order to reduce their hazardous potential.
The (eco)toxicological tests of ionic liquid entities aim for the identification of the individual effects of different headgroups (with identical side chains and anions), anions (with identical cations) and systematic variations of R1, R2etc. at identical head groups and with identical anions. In the following section novel results from different levels of complexity with respect to these questions will be summarised.
Substructures of ionic liquids influence their biological effects
To understand the impact the change of a structural element of an ionic liquid entity has on its (eco)toxicological effects, we asked the following questions:i) How does an increase of the length of a side chain R1, R2etc. affect biological activity, if we keep anion and headgroup constant?
ii) Does the exchange of an anion effect biological activity?
iii) Does the type of headgroup influence the biological activity?
iv) Is a detoxification of ionic liquids by metabolic processes possible?
Side chain length effects
In 2003 we published our first systematic investigations of the influence of an increasing chain length of R1 or R2 of the imidazolium cation moiety on the cytotoxicity in marine bacteria and two types of mammalian cell cultures.14 This effect was also evident in other blood cell lines (U937 and NB4, unpublished results) and in HeLa cells.15 We have checked this “side chain length effect” in the meantime with a multitude of different combinations of cations and anions. It always became evident: the shorter the chain length(s) of side chain(s), the lower the cytotoxicity.Following our concept of a flexible test battery we also investigated the “side chain length effect” at the molecular level, selecting acetylcholine esterase as a model enzyme. This enzyme was chosen because of the similarity of the chemical structure of many ionic liquid cations and acetylcholine. Both are cationic and the positive charge is masked by lipophilic residues. In addition the amino acid side chains forming the wall of the channel-like cleft, in which the acetylcholine molecule is bound are hydrophobic in nature, thus offering potential for hydrophobic interaction with the side chains of an ionic liquid, in case it is recognized and consequently bound to the active site of the enzyme.16 Results for a large set of ionic liquids have been published17 which also show a pronounced “side chain length effect”. The inhibitory potential of an ionic liquid increases with an increase in chain length of the alkyl substituent. The results obtained confirm our T-SAR based choice of the acetylcholine esterase as a test system for ionic liquid toxicity.
Following our concept, as the next level of biological complexity we chose two higher plants which are well established as test organisms to assess ecotoxicological hazards of industrial chemicals: lesser duckweed (Lemna minor), a floating aquatic organism and garden cress (Lepidium sativum), a fast growing terrestrial plant.
Both assays18,19 are much more time consuming than cell culture and enzyme tests. Therefore we chose only two ionic liquids to evaluate the effect of the alkyl chain length: 3-butyl-1-methylimidazolium and 1-octyl-3-methylimidazolium tetrafluoroborates ([bmim][BF4] and [omim][BF4], respectively).
Fig. 4 shows some results obtained with these two ionic liquid entities on the aquatic organism Lemna minor. Compared to the control, the growth (observed as number of foliaceous fronds) in the variant with [omim][BF4] was significantly decreased (87% reduction) at a concentration of 10 mg L−1. No significant decrease (∼4%) was observed at the same concentration of [bmim][BF4]. A significant decrease in number of fronds (60%) was observed with [bmim][BF4] at a concentration of 100 mg L−1.
![Beakers with Lemna minor after 7 days (end of test). Nutrient solution (Steinberg medium) contained [bmim][BF4]
(above) and [omim][BF4]
(below) in concentrations ranging between 0–100 mg L−1.](/image/article/2005/GC/b418518h/b418518h-f4.gif) | ||
| Fig. 4 Beakers with Lemna minor after 7 days (end of test). Nutrient solution (Steinberg medium) contained [bmim][BF4] (above) and [omim][BF4] (below) in concentrations ranging between 0–100 mg L−1. | ||
Fig. 5 demonstrates the influence of the side chain length of both lead chemicals on the terrestrial plant Lepidium sativum. The number of seedlings was significantly decreased in samples with [omim][BF4] at a concentration of 100 mg kg−1 (0% germination), whereas significant effects in samples with [bmim][BF4] were only observed at the highest concentration of 1000 mg kg−1. The average germination in all controls was 95%. Details will be published elsewhere.
![Pots with garden cress (Lepidium sativum) after 21 days (end of test). Soil (Lufa 2.2) was spiked with [bmim][BF4]
(above) and [omim][BF4]
(below) in concentrations ranging between 0–1000 mg kg−1.](/image/article/2005/GC/b418518h/b418518h-f5.gif) | ||
| Fig. 5 Pots with garden cress (Lepidium sativum) after 21 days (end of test). Soil (Lufa 2.2) was spiked with [bmim][BF4] (above) and [omim][BF4] (below) in concentrations ranging between 0–1000 mg kg−1. | ||
Both tests with higher plants confirm again the results of the other tests: the longer the side chain of the ionic liquid, the higher the (eco)toxicity.
We additionally tested the genotoxicity of two ionic liquids using a standard test with mammalian blood cells. The Sister Chromatid Exchange (SCE)20 assay shows the same trend with respect to the alkyl chain length. Again [bmim][BF4] was chosen as lead chemical. This time it was compared to the decyl substituted entity ([dmim][BF4]). In detail, [bmim][BF4] caused no genotoxic effects (for the endpoint SCE aberrations and replication index) within a concentration range from 0 up to 20 µmol L−1. In contrast, [dmim][BF4] showed a dose dependent trend within a concentration range from 0 up to 10 µmol L−1. Increasing doses of [dmim][BF4] caused slightly increasing frequencies of SCE and decreasing fidelity of the target cells as observed via the replication index, although the parameters were not statistically different from the controls (unpublished results).
All results obtained at all biological levels tested so far revealed increasing toxicity (lower EC50 values, EC50 is the effective concentration, where 50% of the cells have died) due to elongated n-alkyl chain length. These results are consistent with published data.14,15,21,22
Anion effects on toxicity of ionic liquid entities
We have recently started a systematic investigation to answer the question of whether exchanging the anion in a given ionic liquid with another will positively or negatively influence the (eco)toxicity of the new ionic liquid entity. Taking into account the number of anions already used in ionic liquids (see Fig. 3), it is necessary to understand the relation between structure and biological effect of the type of anion in an ionic liquid, while starting to use anions as a tool to tune their physical, chemical and technological properties. We started to screen testkits of ionic liquids where the cation structure is kept constant and the anion is varied with the standard WST-cell viability assay. Here we report the first conclusion which can be drawn from these studies.Fig. 6 clearly shows an anion effect on cytotoxicity. We selected those anions which yielded the strongest difference in biological activity. As discussed in a former publication,14 there are several anions which exhibit no significant difference. A publication describing in detail the cytotoxicological results obtained with more than 20 anions and a T-SAR based discussion of possible molecular mechanisms for the observed anion effects is in preparation.
![Influence of different anions ([BF4] and [btfmi]) of ionic liquids on the viability of promyelocytic leukemia rat cells (WST-assay).](/image/article/2005/GC/b418518h/b418518h-f6.gif) | ||
| Fig. 6 Influence of different anions ([BF4] and [btfmi]) of ionic liquids on the viability of promyelocytic leukemia rat cells (WST-assay). | ||
Effects of the cation “headgroup” on the toxicity of ionic liquids
As shown in Fig. 2 several different chemical structures can form the positively charged “headgroup” in ionic liquid entities. With the WST-cell viability assay and the acetylcholine esterase inhibition assay we have screened a range of different substructures. Summarising these data it becomes evident that the chemical nature of a “headgroup” also influences the biological activity, although this influence varies and is in some cases only marginal. Fig. 7 and Fig. 8 demonstrate this observation exemplarily.![Influence of different headgroups ([bmim] and [bmpyr]) of ionic liquids on the viability of promyelocytic leukemia rat cells (WST-assay).](/image/article/2005/GC/b418518h/b418518h-f7.gif) | ||
| Fig. 7 Influence of different headgroups ([bmim] and [bmpyr]) of ionic liquids on the viability of promyelocytic leukemia rat cells (WST-assay). | ||
![Influence of different headgroups ([bmim] and [bmpy]) of ionic liquids on the activity of acetylcholine esterase (WST-assay).](/image/article/2005/GC/b418518h/b418518h-f8.gif) | ||
| Fig. 8 Influence of different headgroups ([bmim] and [bmpy]) of ionic liquids on the activity of acetylcholine esterase (WST-assay). | ||
Chemical transformation of the side chains of ionic liquids may reduce toxicity
In 2003 we published a scheme for a theoretically predicted metabolism of the 1-butyl-3-methylimidazolium [bmim] cation formulated according to an algorithm published by Jastorff and coworkers.8,23 Since investigations in model systems have just started, this working hypothesis has not been experimentally confirmed.We have continued the theoretical formulation of further presumable metabolites of the [bmim] cation and additionally those of the 1-octyl-3-methylimidazolium ([omim]) cation and thus predicted more than 50 chemical entities as candidates for inclusion in the pathways to their complete mineralisation.24
We decided to synthesise some of the most probable predicted metabolites using published standard procedures and determined their cytotoxicity as compared to the parent ionic liquids using the WST-cell viability assay.24 Other “metabolites” like methyl-, butyl- and octylimidazole were purchased commercially. It should be mentioned that some of the metabolites can also be understood as functionalised ionic liquids. The functional groups introduced were a primary alcoholic hydroxyl function, a carbonyl function and the carboxyl group. Indeed some of these intermediate compounds are liquid at room temperature.24
Figs. 9 and 10 show the structures of the potential metabolites selected from the theoretical metabolic schemes.6,24 The corresponding cytotoxicity data (EC50 values) are given under the structural formulae. These data have been obtained with our standard cytotoxicity test and evaluation procedure.14
![Cytotoxicity of [bmim] cation in comparison to some of its hypothetical metabolites.](/image/article/2005/GC/b418518h/b418518h-f9.gif) | ||
| Fig. 9 Cytotoxicity of [bmim] cation in comparison to some of its hypothetical metabolites. | ||
![Cytotoxicity of [omim] cation in comparison to some of its hypothetical metabolites.](/image/article/2005/GC/b418518h/b418518h-f10.gif) | ||
| Fig. 10 Cytotoxicity of [omim] cation in comparison to some of its hypothetical metabolites. | ||
The biological data clearly indicate that the presumable transformation products (“metabolites”) of both ionic liquid entities are less toxic compared to their parent chemicals. Since we have decided not to test cytotoxicity at concentrations higher than 3 mmol L−1, the data given are either below (then exactly) or above that threshold.
Again, a side chain length effect is found for both the alkyl imidazoles (methyl- and butyl- >3000 µM, octyl- = 200 µM) and some of the functionalised cations, e.g. those compounds carrying a terminal hydroxyl function at the butyl (EC50 > 3000 µM) or the octyl moiety (EC50 = 250 µM), respectively. Metabolic transformation to the carboxyl function yields no side chain length effect up to 3000 µM. In conclusion the introduction of polar functional groups into an alkyl chain yields a reduction in cytotoxicity. These results confirm the relation between lipophilicity and cell viability as discussed in a recent publication.14
The need for a purity assessment of ionic liquids
An analytical grade purity of an ionic liquid is necessary, if reliable physical, chemical and biological data are to be determined. However, for technical uses demanding analytical quality is not always feasible. Due to economic reasons only a technical grade may be sustainable. To accept such a “technical grade” quality as sustainable, the nature and the amounts of impurities have to be analysed prior to use and their potential risks for man and the environment have to be assessed. The easiest way to do this is to also use the technical grade ionic liquid as the test chemical for (eco)toxicological testing, and to compare the data obtained with those measured with the analytical grade quality. Discrepancies only have to be evaluated and eliminated by further purification if the technical grade exhibits a higher toxicity.Properties and chemical nature of impurities may also be of importance if the whole life cycle of a chemical product or production process is taken into account, since the impurities may have an impact on the design of waste treatment processes. In summary, the problem of purity as part of the criteria for a sustainable ionic liquid—arising due to multiple usage patterns as products or within processes (synthesis, catalysis, extraction, thermal fluids, new batteries etc.)—in our opinion can most rationally and adequately be solved if a purity check as shown in Fig. 11 is used as the standard of analysis of an ionic liquid. Otherwise side effects of impurities cannot be excluded.21,25
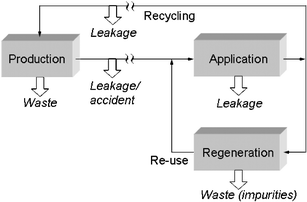 | ||
| Fig. 11 Life cycle of an industrial chemical that satisfies sustainability criteria. The extent of leakage and (unavoidable) waste streams depends highly on the types of product and process. | ||
To check the purity of the ionic liquids for diverse usage patterns, we propose a combination of several methods. Impurities that are volatile at temperatures up to the decomposition of the ionic liquids should be monitored by gas chromatography. Highly volatile compounds can be measured with headspace-GC and less volatile impurities can be detected using liquid injection of sample solutions in lower alcohols. Halide impurities, most frequently stemming from the alkylation of amines or phosphines with alkyl halides in the synthesis of ionic liquids, can be titrated by the Volhard method, lower concentrations in the ppm range can be determined using Nessler cylinders. Due to the influence of traces of water on the physical and chemical properties of ionic liquids, Karl–Fischer titration or GC analysis of water content should be routinely applied. For the quantification of cations and anions, i.e. the main components of structurally similar ionic impurities, reversed-phase or ion exchange HPLC coupled with a suitable detector like UV (in the case of e.g. imidazolium or pyridinium cations)26,27 or conductivity as a more general method can be applied. Recently, a capillary electrophoretic method for resolving selected imidazolium ionic liquid cations has also been presented.28
The combination of all these methods will lead to a reasonably complete picture of impurities of the applied ionic liquid. Furthermore, for studies on bioaccumulation, transformation or persistence, the determination of even low concentrations of an ionic liquid and its metabolites in different media (e.g. water, soil, cell extracts), a tailored sample preparation and chromatographic analysis coupled to structure elucidation methods are a prerequisite. Recently methods for the analysis of imidazolium ionic liquids in aqueous environmental samples have been developed.29
Eco-design of ionic liquids
In 2003 we proposed a first draft for a sustainable product design.6 Here we present a refinement of our approach for discussion within an interested scientific community in academia and industry.The design procedure for “green” ionic liquids currently applied is the following: synthesise a set of ionic liquids, test their biodegradability and (eco)toxicological properties, choose those that show an environmentally benign character, and subsequently try to find a technical application for them. As an alternative concept, we propose an application specific eco-design. This includes the strategy outlined in this publication of defining a toolbox of structural elements valuable for the sustainable design and tuning of ionic liquids in technological applications.
For improving the sustainability of a product the cradle-to-grave impacts of this product on man, environment and profit are to be optimised. Among these impacts are (eco)toxicological hazards as discussed in the sections above and depletion of resources during the whole life-cycle. Depending on the application of the chemical compound and re-use and recycle options, material losses due to leakages and waste streams occur (Fig. 11). These losses, as well as the energy demand of regeneration determine the life cycle inventory (LCI). The results obtained for the environmental categories considered frequently yield conflicting objectives. With economical goals included into consideration the dilemma becomes even worse. If the resulting multi-objective optimization problem can be described mathematically an eco–eco trade-off can solve it.30 In the other case a sequential-iterative procedure, as proposed here, appears to be the best choice.
The general objective in the design of chemicals is to obtain technicophore properties which promise an economical usage of novel compounds. In eco-design there is the environmentally benign character of the substance that has to be considered as an accessory objective. Instead of searching for an eco-solution that satisfies such a second objective, the ecotoxicophore properties as well as the regenerability can be taken as design-constraints. The resulting eco-design scheme is illustrated in Fig. 12.
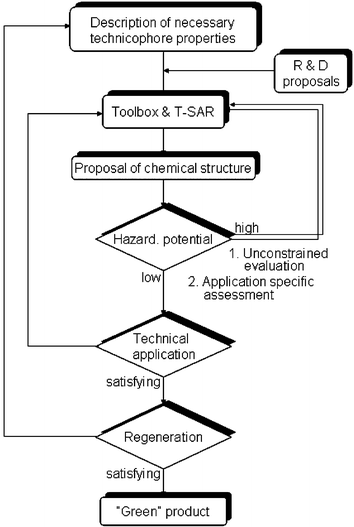 | ||
| Fig. 12 Algorithm for the iterative eco-design procedure of ionic liquids. | ||
In a first loop of the iterative scheme the (eco)toxicity of the proposed compound is evaluated by the screening tests described above. We suppose that for each ionic liquid with a high toxicity another chemical structure with comparable technicophore properties but with a lower toxicity can be found. This means that if a chemical compound is found for example to have a low EC50 value, we will again apply the structure–activity relationships to propose and synthesise a new chemical structure with similar technicophore properties.
However, the risk potential is not determined alone by the toxic character of the compound but by the three issues of environmental relevance, the waste streams of production and regeneration, and the leakage during production, usage and recycling. Obviously the leakage, differs quantitatively with application. During local usage of ionic liquids that serve as thermofluids, for example, leakages might cause soil and groundwater contamination. Used as solvents in two-phase aqueous systems, e.g. in biochemical production processes, ionic liquids are lost with discharged wastewater. On the other hand, if ionic liquids are used on closed chemical production sites, environmental issues are less likely.
To take this application-specific risk potential into account in the risk assessment we propose a second iteration in the eco-design algorithm. Here, the (eco)toxicological hazard potential of the new structural element or compound is assessed for a specific application on the basis of mass balances and indicator values. For the latter the absolute (eco)toxicological characteristics determined in the first iteration are used. The results are compared with constraints, i.e. defined values calculated with respect to no-effect-concentrations. Again, these data are taken from the screening tests.
Finally, if the ionic liquid passed an application test, it is to be tested whether the compound can be recovered after usage e.g. by regeneration, liquid–liquid extraction or nanofiltration. If so, the compound is considered a “green” product.
Conclusions and further research needs
It has been shown that considerable progress has been achieved concerning the assessment of the general biological activity of ionic liquids. This progress has so far focused on screening methods for larger sets of compounds, and on the class of imidazolium based ionic liquids. Some of these have been tested in more complex and resource consuming chronic single-species tests, forming the second level of a flexible biological test battery. The last step would consist of multispecies tests for those chemicals which are of high interest with respect to technological, economical and (eco)toxicological aspects.To obtain more information about the influence of ionic liquids on human health for example after skin contact or during handling of powdered solids additional toxicological data are required. Almost no information about carcinogenicity, genotoxicity or teratological effects is available. First preliminary results with respect to genotoxic effects (SCE-assay) for [bmim][BF4] and [dmim][BF4] were presented here.
More information about the transformation pathways and kinetics of ionic liquids under different environmental conditions and within organisms is necessary. Theoretical metabolism considerations have been presented8,24 and biodegradability studies using the OECD “Closed Bottle Test” were performed for several imidazolium based ionic liquids.31 Further, recent studies investigating the degradation of imidazolium ionic liquids using different oxidation processes showed that degradation efficiency depends on the n-alkyl substituents32 and first sorption results have been published.33 Environmental exposure estimations are severely complicated by the fact that the ionic constituents of ionic liquids tend to be surface active, so conventional bulk phase oriented exposure models are of limited validity.
For further risk assessment studies, more (eco)toxicological data and data on the exposure pathways (for selected technical applications), (bio)transformation and sorption processes as well as bioaccumulation studies are necessary.
In general, more attention has to be drawn to suitable regeneration and/or recycling methods, taking into account the whole life cycle of ionic liquids. The adequate combination of the above elements and a lively communication and discussion of the results provides the opportunity for a truly sustainable development of this fascinating group of chemical substances.
Acknowledgements
The work of this paper is part of the project “Designing Molecular Test Systems for Toxicological and Ecotoxicological Risk Assessment” which is financially supported by the NATO collaborative linkage grant EST.CLG.979251 and of the project “Establishment and Development of the Test Set Ecotoxicity/Toxicity” which is financially supported by the senator for Women, Health, Youth, Social Affairs and Environmental Protection of the Freie Hansestadt Bremen. Support of the Polish Ministry of Scientific Research and Information Technology under grants DS 8000-4-0141-5 and BW 8000-5-0141-5 is also acknowledged. Further we want to thank Christian Jungnickel for improving the English. Special thanks go to Iris Burfeindt and Ute Uebers for carrying out ecotoxicity assays as well as to Anja Müller for checking substance identities with ESI-MS. Dr. N. Ignatiev kindly provided some ionic liquids not commercially available that were synthesised in the lab of Merck KGaA. Contributions of Tanja Juffernholz and Wojciech Mrozik that are not presented in this report are gratefully acknowledged.References
- http://www.un.org/esa/sustdev/documents/agenda21/english/agenda21toc.htm .
- P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- K. R. Seddon, Green Chem., 2002, 4, G35–G36 Search PubMed.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2002 Search PubMed.
- R. D. Rogers and K. Seddon, Ionic Liquids: Industrial Applications for Green Chemistry, American Chemical Society, ACS Symp. Ser. 818, Oxford University Press, Washington (DC), 2002 Search PubMed.
- B. Jastorff, R. Störmann, J. Ranke, K. Mölter, F. Stock, B. Oberheitmann, W. Hoffmann, J. Hoffmann, M. Nüchter, B. Ondruschka and J. Filser, Green Chem., 2003, 5, 136–142 RSC.
- C. A. Doose, J. Ranke, F. Stock, U. Bottin-Weber and B. Jastorff, Green Chem., 2004, 6, 259–266 RSC.
- B. Jastorff, R. Störmann and U. Wölke, Struktur-Wirkungs-Denken in der Chemie—eine Chance für mehr Nachhaltigkeit, Universitätsverlag, Aschenbeck und Isensee, Oldenburg, Germany, 2003 Search PubMed.
- J. Ranke and B. Jastorff, Environ. Sci. Pollut. Res., 2000, 7, 105–114 Search PubMed.
- J. Baker, Ionic Liquids set ideas flowing, Eur. Chem. News, 27.09.–03.10.2004 Search PubMed.
- J. D. Holbrey and K. R. Seddon, Clean Prod. Proc., 1990, 1, 223–226.
- F. Stock, Zelluläre und subzelluäre Testsysteme zur Beurteilung des biologischen Gefährdungspotenzials von Chemikalien im nachhaltigen Chemikaliendesign. Konzept einer Teststrategie und ihre Anwendung in zwei Fallbeispielen, PhD Thesis, University of Bremen, 2004 Search PubMed.
- F. Stock and B. Jastorff, in preparation, 2004.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396–404 CrossRef CAS.
- P. Stepnowski, A. C. Składanowski, A. Ludwiczak and E. Łączyńska, Hum. Exp. Toxicol., 2004, 23(11), 513–517 Search PubMed.
- M. L. Raves, M. Harel, Y. P. Pang, I. Silman, A. P. Kozikowski and J. L. Sussman, Nat. Struct. Biol., 1997, 4, 57 CrossRef CAS.
- F. Stock, J. Hoffmann, J. Ranke, R. Störmann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6, 286–290 RSC.
- DIN EN ISO 5667-16: Water quality—Sampling—Part 16: Guidance on biotesting of samples, International Organization for Standardization, Geneva, 1998.
- ISO 11269-2: Soil Quality—Determination of the effects of pollutants on soil flora—Part 2: Effects of chemicals on the mergence and growth of higher plants, International Organization for Standardization, Geneva, 1995.
- Genetic Toxicology: in vitro Sister Chromatid Exchange Assay in Mammalian Cells, OECD Guideline for testing chemicals 479, Organisation for Economic Co-operation and Development, Paris, 1986.
- R. P. Swatloski, J. D. Holbrey, S. B. Memon, G. A. Caldwell, K. A. Caldwell and R. D. Rogers, Chem. Commun., 2004, 668–669 RSC.
- J. Pernak, K. Sobaszkiewicz and I. Mirksa, Green Chem., 2003, 5, 52–56 RSC.
- R. Störmann and B. Jastorff, Sci. Total Environ., 1993,(Suppl.), 1657–1667.
- S. Stolte, Untersuchungen zum Metabolismus von Ionischen Flüssigkeiten am Beispiel der Imidazol-Derivate, Diploma Thesis, University of Bremen, 2004 Search PubMed.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275–2287 CrossRef CAS.
- P. Stepnowski, A. Müller, P. Behrend, J. Ranke, J. Hoffmann and B. Jastorff, J. Chromatogr., A, 2003, 993, 173–178 CrossRef CAS.
- P. Stepnowski and W. Mrozik, J. Sep. Sci., 2005, 28(2), 149–154 Search PubMed.
- M. Markuszewski, P. Stepnowski and M. Marszałł, Electrophoresis, 2004, 25, 3450–3454 CrossRef CAS.
- P. Stepnowski, Anal. Bioanal. Chem., 2005, 381(1), 189–193 CrossRef CAS.
- P. Erol and J. Thöming, J. Cleaner Prod., 2005 Search PubMed (in press).
- N. Gathergood, M. T. Garcia and P. J. Scammells, Green Chem., 2004, 6, 166–175 RSC.
- P. Stepnowski and A. Zaleska, J. Photochem. Photobiol., A: Chem., 2004, 170, 45–50.
- P. Stepnowski, Preliminary assessment of the sorption of some alkyl imidazolium cations as used in ionic liquids to soils and sediments, Aust. J. Chem., 58, 170–173 Search PubMed.
Footnote |
| † This work was presented at the Green Solvents for Synthesis Meeting, held in Bruchsal, Germany, 3–6 October 2004. |
| This journal is © The Royal Society of Chemistry 2005 |
