High performance NMR in ionic liquids†‡
Ralf
Giernoth
*,
Dennis
Bankmann
and
Nils
Schlörer
Universität zu Köln, Institut für Organische Chemie, Greinstr. 4, 50939 Köln, Germany. E-mail: Ralf.Giernoth@uni-koeln.de
First published on 14th March 2005
Abstract
Nuclear magnetic resonance techniques for investigations of and in neat ionic liquids have been developed. After thorough optimisation, a resolution comparable to classical solvents is achieved. The technique is usable for a wide range of ILs. Observed nuclei are 1H and 13C and potentially 2H and 19F. Measurements of T1 values show multipulse experiments to be feasible.
 From left to right: Ralf Giernoth, Sven Arenz, Matthias S. Krumm, Dennis Bankmann, and our close cooperation partner Nils Schlörer. | Ralf Giernoth (born in 1970) received his PhD from the University of Bonn (Germany) under the supervision of Prof. Joachim Bargon. After two years of post-doctoral research at the University of Oxford (UK) with Dr. John M. Brown as a BASF research fellow, he moved to Cologne (Germany). With an Emmy Noether fellowship (DFG) for young researchers he started to build up his own independent group. His research interests include the synthesis and application of ionic liquids, especially for transition metal catalysis, and the development of in situ spectroscopic methods. |
Introduction
The interest in ionic liquids1 (ILs) as novel solvents is ever increasing. However, to date only a very limited range of analytical techniques for everyday use is available. While the purity of the IL itself can be checked by standard analytical methods like NMR in deuterated solvents and mass spectrometry, the bulk structure of the solvent and its participation in reactions is still not well understood. There is therefore a need for in situ techniques.First attempts to characterize the liquid state structure of ILs have appeared in the literature, employing mass spectrometry,2–4 infrared spectroscopy,5,6 and NMR. Magnetic resonance measurements have been applied to investigate self-diffusion coefficients and viscosities,7 ion-pair formation,8 proton conductance,9 and the structural consequences of water traces.10 However, the few spectra published show limited resolution or are not spectra of neat ILs without any other solvent. Furthermore, the use of deuterated substrates for in situ investigations in protonated ILs has been demonstrated.11
A range of experiments has been performed on first generation ionic liquids containing chloroaluminate anions.12–17 These results however do not directly relate to modern ILs.
While these investigations have shed light on some structural aspects, a more systematic approach that is compatible with a wide range of ILs is needed to make such methods routinely available to non-spectroscopists. NMR spectroscopy in ILs should provide reproducible results, high sensitivity, and should not require deuterated ILs or substrates for the majority of applications.
The implementation of high resolution nuclear magnetic resonance (NMR) spectroscopy in ionic liquids may help to reveal liquid state structure and reactivity of the solvent, allows for process and purity control in industrial environments, and opens a way towards in situ investigations of reactions in ionic liquids.
Results
Setup and lock
As ionic liquids are already quite expensive solvent systems, the use of deuterated ILs is not desirable. The absence of deuteration in commonly used ionic liquids therefore prohibits the use of solvent deuterium nuclei for locking purposes. While the addition of deuterated locking additives to the liquid is possible, these will disturb the bulk structure of the IL or influence reactions. Of course, it is possible to use a coaxial tube insert containing a deuterated substance to lock via the deuterium channel, but this practice frequently makes the lock signal level a bad indicator for the shim quality.The fluorine nuclei of the two common IL anions Tf2N− and BF4− provide an internal 19F lock signal that is more susceptible to changes in field homogeneity. Furthermore, this practice allows for the acquisition of 2H spectra with 19F lock, which is very helpful for mechanistic studies using H/D labelling, but requires a probe with a separate fluorine channel and a corresponding lock unit and preamplifier.
On standard probes, optimum matching may not be achievable due to the differences in physical properties between ionic liquids and standard laboratory solvents. The mismatching will negatively affect sensitivity, pulse lengths and signal quality. For these reasons, we employ a Bruker TXO probe with 1H, 2H, 13C and 19F channels specifically matched to ionic liquids.
Experiments in ionic liquids require comparatively high pulse power levels and lengths as well as high lock power. In our setup, typical 90° pulses for the proton channel at typical pulse power levels were around 16 µs on a 5 mm broadband inverse probe (as compared to 9–10 µs for common NMR solvents). Carbon pulse lengths were typically about 15 µs. Using the 10 mm TXO probe, pulse lengths were about 28 µs for protons and 20 µs for carbon nuclei. All of these values are well within tolerable limits for multipulse experiments. We attribute the high pulse lengths to a comparatively strong absorption of radio frequency radiation by the ionic liquid. During the course of the experiments no sample heating has been observed, however.
Referencing
When applied in the course of in situ studies, the chemical shift axis of the NMR spectrum may be conveniently calibrated via an arbitrary internal standard. The direct addition of tetramethylsilane (TMS) to the IL as a proton and carbon shift standard is undesirable, though, as it may interact with the system under investigation. A coaxial glass insert containing pure TMS proved suitable for 1H and 13C external referencing of 19F locked acquisitions, likewise a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of TMS and acetone-d6 for 2H locking and proton referencing. Due to the attenuation of signals through the ionic liquid medium, the use of a standard solution (e.g. 1% TMS in chloroform-d) is not helpful. To compensate for changes in dielectric properties and obtain correct shift values, further referencing against the standards as defined by IUPAC is necessary.18
1 mixture of TMS and acetone-d6 for 2H locking and proton referencing. Due to the attenuation of signals through the ionic liquid medium, the use of a standard solution (e.g. 1% TMS in chloroform-d) is not helpful. To compensate for changes in dielectric properties and obtain correct shift values, further referencing against the standards as defined by IUPAC is necessary.18
Relaxation
To assess the feasibility of multipulse experiments, proton T1 values have been determined for a range of imidazolium type ionic liquids with Tf2N− and BF4− anions via inversion recovery experiments. Taking into consideration the typical pulse lengths mentioned above, the results in Fig. 1 show multipulse sequences to be possible. However, T1 based suppression of the solvent signals from the spectrum, as would be desirable for routine spectroscopy, is precluded by the similarity of solvent and solute relaxation times.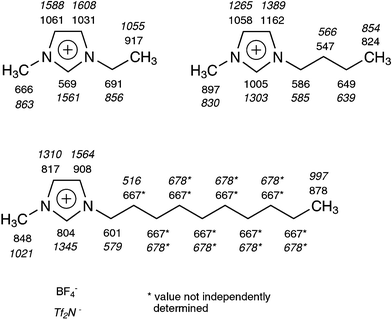 | ||
| Fig. 1 Proton relaxation data in milliseconds acquired on a 10 mm TXO probe with 19F lock. Values marked with an asterisk have been determined for multiple protons with overlapping signals. | ||
An important trend to notice here is the reduction of T1 values of the imidazolium ring protons when going from Tf2N− to BF4− anions. This is in good agreement with the observation of H-bonding of BF4− to all three ring protons.8,10,17,19
1D spectra of common ILs
The spectra of neat ionic liquids closely resemble those measured in standard NMR solvents. Fig. 2 shows the experimental proton spectrum of [bmim][Tf2N].![1H spectrum of neat [bmim][Tf2N] at 298 K acquired on a TXO probe with 19F lock.](/image/article/2005/GC/b417783e/b417783e-f2.gif) | ||
| Fig. 2 1H spectrum of neat [bmim][Tf2N] at 298 K acquired on a TXO probe with 19F lock. | ||
The 13C spectra show a similar appearance and high sensitivity. The acquisition of high S/N-ratio spectra of the IL is possible with less than 8 scans. In all experiments, the CF3 quartet of the Tf2N− anions is clearly visible (Fig. 3).
![13C spectrum of neat [bmim][Tf2N] at 298 K acquired on a TXO probe with 19F lock.](/image/article/2005/GC/b417783e/b417783e-f3.gif) | ||
| Fig. 3 13C spectrum of neat [bmim][Tf2N] at 298 K acquired on a TXO probe with 19F lock. | ||
Resolution and performance
After careful optimisation of shims and pulse lengths, we were able to attain high resolution and good lineshapes routinely. The typical signal widths of about 3–5 Hz (full width at half maximum) in proton spectra at room temperature are still somewhat higher than in classical solvents, which we presume is a result of the higher viscosity of ionic liquids. Performing experiments at temperatures between 278 K and 338 K (Fig. 4) accordingly show decreasing line width with increasing temperature.![The CH2–CH3 signal of [bmim][Tf2N] as a function of the sample temperature. The spectra were acquired with 2H lock on a 5 mm BBI probe.](/image/article/2005/GC/b417783e/b417783e-f4.gif) | ||
| Fig. 4 The CH2–CH3 signal of [bmim][Tf2N] as a function of the sample temperature. The spectra were acquired with 2H lock on a 5 mm BBI probe. | ||
Solutes in ionic liquids
As a test substance, we dissolved a drop of ethanol in the ionic liquid [bmim][Tf2N]. As can be seen from the spectrum in Fig. 5, the CH2 and CH3 groups are visible and mostly resolved in the spectrum. The alcohol proton is not visible, however. In the carbon spectrum, both ethanol signals are clearly visible (see supporting information‡).![Proton spectrum of [bmim][Tf2N] containing one drop of ethanol. The spectrum was acquired with 2H lock on a 5 mm BBI probe.](/image/article/2005/GC/b417783e/b417783e-f5.gif) | ||
| Fig. 5 Proton spectrum of [bmim][Tf2N] containing one drop of ethanol. The spectrum was acquired with 2H lock on a 5 mm BBI probe. | ||
This experiment shows that the strong signals from the undeuterated solvent do not preclude observation of solutes. The solute signals appear with similar resolution to those of the solvent. Optimisation is expected to increase the resolution and sensitivity further. With the advent of solvent suppression techniques, even weaker signals or signals which are isochronous to solvent signals will become available for observation.
Advanced experiments
We were able to carry out 2-dimensional experiments like COSY, HETCOR and gradient-selected experiments like HMQC on a range of neat ILs. The spectra are available in the supporting information‡ for reasons of brevity, as they closely resemble those measured in standard solvents. First multipulse experiments focusing on solutes in ILs could also be realised. The results parallel those in standard solvents. Fig. 6 shows the H,H-COSY spectrum of [bmim][Tf2N] containing a drop of ethanol. The crosspeaks for the coupling between the CH2 and CH3 groups of ethanol can be clearly discerned from the spectrum.![Gradient COSY spectrum of [bmim][Tf2N] containing a drop of ethanol. The spectrum was acquired with 2H lock on a 5 mm BBI probe. The dashed lines indicate the ethanol crosspeaks.](/image/article/2005/GC/b417783e/b417783e-f6.gif) | ||
| Fig. 6 Gradient COSY spectrum of [bmim][Tf2N] containing a drop of ethanol. The spectrum was acquired with 2H lock on a 5 mm BBI probe. The dashed lines indicate the ethanol crosspeaks. | ||
Conclusions
The use of NMR as an in situ technique in ionic liquids has been systematically investigated and established. Resolution and appearance of 1H and 13C spectra of neat ILs have been shown to be similar to those in classical deuterated solvents. Thus, ionic liquids can be regarded as acceptable NMR solvents. The synthesis of deuterated ILs for NMR spectroscopy was shown to be avoidable.We believe that NMR spectroscopy has a high potential for investigating the liquid phase structure of ionic liquids as well as in situ investigations of reactions in ionic liquids, especially in cases where the solvent participates. Our current work focuses on advanced NMR techniques including heteronuclear NOE experiments and solvent signal suppression techniques.
Experimental
The fluorine locked experiments were carried out on a Bruker DRX-500 spectrometer (1H base frequency 500 MHz) using a Bruker 10 mm TXO 1H/19F/13C probe with 1H, 2H, 13C and 19F channels. The probe is specifically matched to ionic liquids. Additional experiments were performed on a Bruker 5 mm BBI 1H-BB-D Z-GRD probe with gradient unit. For the one-dimensional experiments the 90° pulse angles were determined and subsequently used in standard pulse sequences. The correlation experiments were performed using standard gradient-selected COSY and HMQC pulse sequences. Unless noted otherwise, the experiments were performed at 298 K using 5 mm NMR tubes (Norell 508-UP). The locking additives were taken from fresh bottles and were injected into 5 mm coaxial inserts under vacuum, frozen with liquid nitrogen and subsequently sealed under vacuum.The ionic liquids were synthesised according to known literature procedures20 and dried for 5 h at 70 °C under high vacuum.
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft for financial support of this work through the Emmy Noether programme.References
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2003 Search PubMed.
- P. Dyson, J. McIndoe and D. Zhao, Chem. Commun., 2003, 508–509 RSC.
- P. Dyson, I. Khalaila, S. Luettgen, J. McIndoe and D. Zhao, Chem. Commun., 2004, 2204–2205 RSC.
- G. Jackson and D. Duckworth, Chem. Commun., 2004, 522–523 RSC.
- C. D. Tran, S. H. D. P. Lacerda and D. Oliveira, Appl. Spectrosc., 2003, 57, 152–157 CrossRef CAS.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- A. Noda, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2001, 105, 4603–4610 CrossRef CAS.
- J.-F. Huang, P.-Y. Chen, I.-W. Sun and S. P. Wang, Inorg. Chim. Acta, 2001, 320, 7–11 CrossRef CAS.
- A. Noda, A. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- A. Mele, C. D. Tran and S. H. D. P. Lacerda, Angew. Chem., 2003, 115, 4500–4502 CrossRef; A. Mele, C. D. Tran and S. H. D. P. Lacerda, Angew. Chem., Int. Ed., 2003, 42, 4364–4366 CrossRef CAS.
- A. Durazo and M. M. Abu-Omar, Chem. Commun., 2002, 66–67 RSC.
- C. E. Keller and W. R. Carper, J. Phys. Chem., 1994, 98, 6865–6869 CrossRef CAS.
- P. C. Trulove, D. K. Sukumaran and R. Osteryoung, Inorg. Chem., 1993, 32, 4396–4401 CrossRef CAS.
- Y. S. Fung and S. M. Chau, Inorg. Chem., 1995, 34, 2371–2376 CrossRef CAS.
- J. L. E. Campbell, K. E. Johnson and J. R. Torkelson, Inorg. Chem., 1994, 33, 3340–3345 CrossRef CAS.
- S. Takahashi, M. L. Saboungi, R. J. Klingler, M. J. Chen and J. W. Rathke, J. Chem. Soc., Faraday Trans., 1993, 89, 3591–3595 RSC.
- A. Avent, P. Chaloner, M. Day, K. Seddon and T. Welton, J. Chem. Soc., Dalton Trans., 1994, 3405–3413 RSC.
- R. K. Harris, E. D. Becker, S. M. C. D. Menezes, R. Goodfellow and P. Granger, Pure Appl. Chem., 2001, 73, 1795–1818 CrossRef CAS.
- J. Antony, D. Mertens, A. Dölle, P. Wasserscheid and W. R. Carper, ChemPhysChem, 2003, 4, 588–594 CAS.
- R. Giernoth and M. Krumm, Adv. Synth. Catal., 2004, 346, 989–992 CrossRef CAS.
Footnotes |
| † This work was presented at the Green Solvents for Synthesis Meeting, held in Bruchsal, Germany, 3–6 October 2004. |
| ‡ Electronic supplementary information (ESI) available: 13C spectrum of ethanol in [bmim][Tf2N] and gradient-selected spectra of ILs. See http://www.rsc.org/suppdata/gc/b4/b417783e/ |
| This journal is © The Royal Society of Chemistry 2005 |
