The current status of fuel cell technology for mobile and stationary applications
Frank
de Bruijn
ECN Fuel Cell Technology, PO Box 1 1755 ZG Petten, The Netherlands. E-mail: debruijn@ecn.nl
First published on 10th February 2005
Abstract
This review of fuel cell technology gives an overview on the status of low and high temperature fuel cells, both on materials and component level as well as on a system level. Their application in transport and the combined generation of heat and power is discussed in relation to their environmental benefits.
 Frank de Bruijn Frank de Bruijn | Frank de Bruijn, born in 1966, is leading the Fuel Cell Technology unit at the Energy research Centre of the Netherlands. He studied electrochemistry in Utrecht and received his PhD in Chemical Engineering in Eindhoven. In 1996, he joined ECN where he worked on Fuel Cells and Applied Catalysis. At ECN, more than 50 people are working on the research and development of materials and components for fuel cell systems and on system integration. |
1. Introduction
Fuel cells are generally considered as a clean, efficient and silent technology that can produce electricity and heat from fossil fuels, biofuels as well as hydrogen produced from renewable energy sources such as wind energy and solar energy. The expectations with respect to their commercial introduction in transport as early as 20031–3 and stationary applications by 20011,4,5 held since the mid 1990s have not yet been realised. The main hurdles preventing commercial introduction still are too high cost, lack of durability, too high system complexity and a lack of fuel infrastructure. To better understand the issues to be discussed in this review on fuel cells, the basic principles of fuel cells are explained in this introduction.1.1. Fuel cell principle and fuel cell types
The basic principle of the fuel cell is illustrated in Fig. 1. The core of each fuel cell consists of an electrolyte and two electrodes. At the negative anode, a fuel such as hydrogen is being oxidized, while at the positive cathode, oxygen is reduced. Ions are transported through the electrolyte from one side to the other. The type of electrolyte determines the temperature window of operation. This window of operation in its turn determines the catalyst that can be used, and the purity of the fuel to be used. At open circuit, the voltage of a hydrogen–oxygen fuel cell is 1.23 V at 298 K. Under load conditions, the cell voltage is between 0.5 and 1 V. | ||
| Fig. 1 Basic principle of a fuel cell, for the case where a proton-conducting electrolyte is used as separator. | ||
Six types of fuel cells have evolved in the past decades. A brief summary of these six fuel cell types is given below. This review focuses on the PEMFC and SOFC, as worldwide the most research and development is performed on these two fuel cells.
1.2. Fuel cell setup: from single cell to systems
A single fuel cell, as displayed in Fig. 2, produces the power, which results from the area × the current density of the cell × the cell voltage. The typical cell voltage under load conditions amounts to 0.7 V, which is too low for practical applications.
 | ||
| Fig. 2 Fuel cell components of a single cell. | ||
 | ||
| Fig. 3 Schematic, simplified overview of a fuel cell stack. | ||
 | ||
| Fig. 4 Schematic, simplified overview of a PEM fuel cell system. | ||
In low temperature fuel cells, except the DMFC, hydrogen is oxidized at the anode to protons. The hydrogen can either be fed from a hydrogen storage container, or produced from another fuel in a so-called fuel processor. Generally, hydrocarbons or alcohols are used as fuels to feed fuel processors. The complexity of the fuel processing depends strongly on the fuel cell type and the primary fuel. In high temperature fuel cells, such as the MCFC and SOFC, fuel processing can be done in the fuel cell itself. This process is referred to as internal reforming. In section 4 fuel processing will be further discussed.
The air pressure needs to be elevated from ambient pressure up to a level, which depends on the operation pressure and the pressure drop in the complete system. This can range from a gauge pressure of 100 mbar to several bars. The power of the fuel cell stack generally increases with increasing pressure; the parasitic loss of the compression however increases as well.
The voltage of the fuel cell stack is the product of the number of cells × the individual cell voltage, which is typically 0.6–0.7 V DC. For mobile applications, the voltage should be increased to several hundred Volts and conditioned to the needs of the electric motor. For stationary applications, generally AC voltage is needed, which requires a DC/AC inverter.
1.3. System efficiency
The efficiency of the fuel cell stack (EffFC), the hydrogen production (EffH2), the utilization of the hydrogen (UtilH2) and power consumed by the balance of plant components (PowerBOC) determine the total system efficiency:| Effel, sys = EffH2 × EffFC × UtilH2 × (1−(PowerBOC/PowerFuel cell system)) |
In all cases, the full fuel to electricity chain efficiency should be considered, and not only the efficiency of the fuel cell system itself. Especially the production and transportation of hydrogen can cause considerable loss of energy before the hydrogen is converted in the fuel cell system.
The fuel cell efficiency for hydrogen–oxygen fuel cells, based on the higher heating value of hydrogen, which is 142 MJ kg−1, can be obtained by dividing the cell voltage at operation by 1.48 V. The maximum theoretical efficiency of a hydrogen–oxygen fuel cell at 298 K and atmospheric pressure is 1.23/1.48 = 0.83.9 Hydrogen–oxygen fuel cells operated at 0.7 V thus have an electrical efficiency of 0.47. Often, efficiencies in literature are referred to as Lower Heating Value (LHV) efficiencies. As the Lower Heating Value of hydrogen amounts to 120 MJ kg−1, these LHV efficiencies are 1.18 times higher than the HHV efficiency.10
2. Current status of PEM fuel cells
The Proton Exchange Membrane Fuel Cell (PEMFC) is the most widely used fuel cell in transport applications. Since 2000, more than 90% of all fuel cell vehicles on the road have been equipped with a PEMFC.11 The low temperature of operation and its high power density both at its operating temperature as well as during start-up, make it the most suitable fuel cell for transport applications.The large research and development efforts put into the PEMFC, combined with the potentially large production volumes and low cost targets associated with the automotive markets, have made the PEMFC an attractive candidate for application in stationary applications as well.
2.1 Electrolytic membranes
The vast majority of PEMFCs use a perfluorosulfonic acid–tetrafluoroethylene copolymer as membrane material. Membranes in PEM fuel cells nowadays have a thickness between 30 µm and 100 µm, depending on whether they are reinforced or not. The main supplier of the non-reinforced membranes is Dupont, selling the perfluorosulfonic acid membrane under the trade name Nafion.12 It can be operated at temperatures between 0 and 80–90 °C, depending on the cell pressure. Dehydration of this type of membrane has to be prevented, as its conductivity, typically 0.1 S cm−1 at normal operating conditions, dramatically decreases when its water content drops below full saturation.13 The requirement for full hydration of the membrane leads to a fuel cell system operation with very complicated water and heat management, especially at low operating pressures.14All PEM fuel cells in commercial development use this membrane or one of its analogues. One of these analogues is the Aciplex membrane, commercialized by Asahi Chemical. Reinforced membranes are commercialized by Gore, and consist of a porous polytetrafluoroethylene (PTFE) matrix, the pores of which are filled with a Nafion-type electrolyte.15,16 The benefit of this composite membrane concept is that the thickness of the membrane can be very small, typically 30 µm or even less, due to the strength of the PTFE matrix. Thin membranes have a very low resistance15 and it is much easier to keep them hydrated than a thick membrane.
Alternative membranes for the PEMFC have been developed initially mainly for reasons of lower cost. All membranes used for PEM fuel cells to be operated at temperatures below 100 °C, contain sulfonic acid groups for proton conduction. A few examples are sulfonated polyethersulfone,17,18 sulfonated polyetheretherketone19 and sulfonated α,β,β-trifluorostyrene.18,20 It has proven to be difficult to develop cheaper alternatives that can meet the requirements on durability.21,22 Peroxy radicals formed in the oxygen reduction reaction make most polymers with C–H bonds susceptible to degradation. The fully fluorinated Nafion membrane or its analogues can stand this harsh environment for a long time, although some fluoride is lost.12 Modifications of Nafion can lead to lower fluoride losses.12
Two important factors have led to an increase in research and development effort towards high temperature (120–180 °C) proton conducting membranes: too low CO tolerance and poor heat transfer on a system level associated with the PEMFC being operated at temperatures between 70 and 80 °C.
CO tolerance means that low concentrations of CO in the fuel cell feed does not lead to an extreme loss in fuel cell performance. In the state-of-the-art PEMFC, a CO level of 10 ppm leads to a loss in fuel cell power of 20–50%, depending on the type of catalyst being used at the anode, and the conditions with respect to fuel humidity and pressure. Complete removal of CO in the fuel processor is only effective in the complete absence of CO2, as the reverse water gas shift reaction between CO2 and H2 leads to formation of CO and water.23
Much work has been devoted to the use of phosphoric acid doped polybenzimidazole (PBI) membranes, which can be operated at a temperature between 125 °C and 200 °C.24 This is immediately the drawback of this type of membrane: the proton conductivity at temperatures below 100 °C is too low, such that the cold-start properties of the classic PEMFC are lost.
The benefit of fuel cell operation at temperatures above 100 °C with respect to CO tolerance is demonstrated by Li et al..25 The use of phosphoric acid doped PBI enabled them to investigate the influence of temperature on the CO tolerance of the PEMFC in the temperature range between 125 °C and 200 °C. Even when using unalloyed platinum catalysts, the effect of CO is very limited. At 125 °C, the effect of 1000 ppm CO is already minor. At 200 °C, 3% CO can be tolerated with a very small drop in performance. The power output at 200 °C is around 0.5 W cm−2, at 125 °C it is less than 0.25 W cm−2. Compared to a Nafion based PEMFC, this means that while at 200 °C power density is satisfactory, at temperatures below 125 °C it is too low for practical application.
Extensive reviews of alternative membranes under development for high temperature operation have been published by Savogado26 and Li et al.27 It should be noted that it will take a long time before alternative membranes will be used in practical fuel cell systems which have comparable performance and proven endurance compared to Nafion based fuel cell systems.
The high cost of the perfluorosulfonic acid–tetrafluoroethylene copolymer membranes has been the driving force for the development of cheap alternatives. The cost level of these Nafion membranes used to be in the order of $800 m−2.28 Developers of alternative membranes were aiming at a cost level of $30–50 m−2.28,29 Probably, the development of cheap alternatives for Nafion has been frustrated by the forecast that the cost level of Nafion would drop when the markets would demand it to $50 m−2.22,30 At a cell power density of 0.5 W cm−2, and an active area/total area ratio of 80%, a cost level of $50 m−2 corresponds to $12.5 kW−1.
2.2. Catalysts and electrodes
Only platinum based catalysts have sufficient activity in the 80–100 °C temperature range to meet power density targets set for mobile and stationary applications. 20–40 wt% Noble metal catalysts are commonly used. Electrode layer thickness amounts to 10 µm, in order to minimize the transport resistance for reactants and protons.31For the anode, the composition depends on the fuel being used. When hydrogen is used with CO levels below the ppm range and with CO2 levels not exceeding the percentage level, platinum on carbon suffices. Noble metal content at the anode is typically 0.2 mg per cm2 active cell area. Even lower noble metal contents have been reported.32,33 Lowering the platinum loading at the anode to 0.05 mg cm−2 does lead to a negligible reduction in cell power density.33 Using such a low anode loading would result in platinum usage for the anode of 0.08 g Pt kWe−1.
When reformed hydrocarbons, alcohols or ethers are being used as hydrogen fuel, CO levels of 10 ppm and higher are commonly present, as well as CO2. Platinum catalysts are severely poisoned by CO. PtRu and PtMo show superior tolerance towards CO compared to unalloyed Pt.34 In addition, CO2 present in the reformate in concentrations of 10–25%, leads to CO by the so-called reversed water gas shift reaction.35 Thermodynamically, a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2
1 H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture at the PEM fuel cell operating conditions is in equilibrium with 25 ppm CO or higher, depending on the water content, pressure and temperature.23
CO2 mixture at the PEM fuel cell operating conditions is in equilibrium with 25 ppm CO or higher, depending on the water content, pressure and temperature.23
The formation of CO from CO2 and its effect can be mitigated by alloying platinum with ruthenium.23 However, the power density on pure or nitrogen-diluted hydrogen cannot be matched as long as CO is part of the fuel. This leads generally to lower fuel cell efficiency and to higher noble metal contents. The negative impact of carbon monoxide can in many systems be mitigated by dosing a small amount of air, typically 2%,36 to the reformate stream. By this, the CO is oxidized to CO2, which at the same concentration level has a much smaller impact on the fuel cell performance than CO.37 With respect to the noble metal loading under reformate conditions, a minimum of 0.2 mg cm−2 gives acceptable performance when 2% air bleeding is applied in the presence of 100 ppm CO. Lower noble metal loadings lead to an extra voltage loss of 0.2 V.32
PEM fuel cells using electrodes containing 0.18 mg cm−2 Ru and 0.02 mg cm−2 Pt have been reported to give an acceptable power density of 0.3 W cm−2 both on hydrogen as well on hydrogen with 50 ppm CO with an air bleed. The durability of the fuel cell using these low noble metal loadings is promising, but needs further improvement.33 State-of-the-art PtRu anodes would result in 0.3 g PtRu kWe−1, at 1 A cm−2 at 0.65 V. Note that the air-bleed will result in a lower fuel cell efficiency, as the air not used for CO oxidation will lead to the non-electrochemical oxidation of hydrogen.
At the cathode, platinum on carbon is used. Platinum alloys are under investigation, as especially PtCr shows improved performance.38 The gains are however minor, and up till now fuel cell stacks generally do not make use of cathode catalysts other than unalloyed platinum. The noble metal content amounts to 0.2–0.4 mg cm−2. To prevent voltage losses at the cathode, electrode optimization is important, to prevent mass transport limitation of oxygen. Inefficient removal of product water has an extremely strong influence on the fuel cell performance as it can completely block the transport of oxygen to the reaction interface.39 When an optimized electrode structure is used, 0.2 mg cm−2 Pt gives acceptable PEMFC performance, resulting in 0.32 g Pt kWe−1.32
For hydrogen–air systems, noble metal loading could be as low as 0.4 g Pt kWe−1. For reformate–air systems the noble metal loading would increase to 0.3 g PtRu and 0.3 g Pt kWe−1. These required noble metal loadings are based on short-term performance measurements and not on full life endurance tests. Especially contaminants in reformate systems can lead to catalyst poisoning. Higher noble metal loadings can in the case of poisoning extend fuel cell life considerably.
Noble metal usage in the fuel cell stack at low noble metal loading amounts to $10 kWe−1 for hydrogen systems and $15 kWe−1 for reformate systems, using $25 per gram of noble metal. Catalyst and electrode manufacturing cost will lead to additional costs. Due to the relatively high noble metal loadings of 20–40 wt%, these additional costs are expected to be relatively insignificant.
2.3 Flow plates
The component which has the highest impact on the weight and volume of the fuel cell stack, is the flow plate. Whereas the flow plates used to be made from high-density graphite, nowadays the material of choice is a mouldable graphite–polymer composite material. Although the latter has a somewhat lower conductivity, it enables due to its higher mechanical strength and its higher flexibility the use of plates with lower thickness than when using pure graphite plates. This directly leads to reduction of stack weight and volume. A major advantage of polymer–graphite plates is the fact that they can be manufactured by means of injection moulding.40An alternative to graphite and polymer–graphite material plates are metal plates.41–43 The main advantage of metal plates is the fact that very thin metal sheets can be used, and mass manufacturing techniques are available for forming flow patterns in these sheets. The power density of stacks based on metal bipolar plates has been demonstrated to be as high as 1.6 kW l−1.44 To resist the corrosive environment of the PEMFC, either a special stainless steel alloy,41,44 or coated plates44,45 have to be used.
For very large quantities, the manufacturing procedure for steel plates, being stamping, could be cheaper than the moulding procedure. It could very well be that the power density requested by automotive applications, combined with it's relatively short operating lifetime of typically 3000–5000 hours for passenger cars, leads to the use of metal plates in automotive fuel cells. In stationary applications, where operating lifetime should exceed 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours, and where a high power density is not as stringent as in automotive applications, it is more likely that mouldable flow plates will be preferred.
000 hours, and where a high power density is not as stringent as in automotive applications, it is more likely that mouldable flow plates will be preferred.
Moulded graphite–polymer composite plates can in large quantities be manufactured at a cost of €1.4 per plate of 625 cm2, 46 or €0.7 per plate of 200 cm2, 40 corresponding to approximately €8–12 kW−1, at a cell power density of 0.5 W cm−2.
Graphite–polymer bipolar plate costs calculated in the DoE Hydrogen and Fuel Cell Program add up to $46 m−2, corresponding to $18 kWe−1 at 0.5 W cm−2. 47
For metal plates, costs are calculated in the DoE program to amount to $117–$171 m−2, corresponding to $45–$67 kWe−1 depending on whether SS316 or SS904L is used as base material.47 Probably these figures for metal plates are overestimated, as in the first place the plate thickness assumed is 1 mm, where it can be as thin as 0.1–0.25 mm. Second, the coating costs are assumed to cause more than 50% of the total plate costs, $63 m−2, leaving much room for further cost reduction.
2.4 PEMFC—state of the art performance
Table 1 summarizes the state-of-the-art performance of PEM fuel cells under different conditions. Cell power densities of 0.5 W cm−2 at a cell voltage of 0.7 V can be regarded as state-of-the-art for PEM fuel cells operated at temperatures of 80 °C and lower, at a pressure of 1.5 bar g. Lower pressures render lower power densities.| Cell power density/W cm−2 at 0.7 Vcell | Company | Ref | |
|---|---|---|---|
| H2–O2, 0.5 bar g | 0.84 | Johnson Matthey | 48 |
| H2–air ambient pressure | 0.56 | UTC Fuel Cells | 49 |
| 0.35 | Umicore | 15 | |
| H2–air, 0.5 bar g | 0.42 | Johnson Matthey | 48 |
| H2–air, 1.5 bar g | 0.5 | General Motors | 32 |
| 0.7 | Gore | 50 | |
| Reformate + air bleed–air 1.5 bar g | 0.5 | General Motors | 32 |
PEMFC stacks have been developed for both transport as well as stationary applications. In the case of transport, the design is focused on integrating the fuel cell stack into passenger vehicles, such that a stack producing typically 70 kW or more fits in the floor or under the hood of the car. The power density of these stacks is typically above 1 kW l−1, when operated on hydrogen at a pressure of 1 to 2 bar g and 80 °C.44,51,52Table 2 gives an overview of state-of-the-art stack performance levels.
Stack technology has been improved considerably during the past decade. Whereas in the early 1990s stack power density was 0.2 kW l−154,55 nowadays stack power densities over 1.5 kW l−1 are realized by several companies.
The power density of fuel cell stacks developed for automotive applications and operated on hydrogen is significantly larger than those developed for stationary applications. First, stationary systems are generally operated and designed for lower pressures, below 0.5 bar g. Second, stationary systems are operated on natural gas reformate with hydrogen concentration between 40–75%, depending on the reformer technology used. Third, efficiency requirements are more important and volume requirements less stringent than for automotive applications, so cells are operated at higher cell voltage and lower power density. Especially for micro combined heat and power systems of 1–5 kWe, the end plates, current collector plates and tie rods make a relatively large contribution to the total stack volume and weight.
2.5. PEMFC durability
When accepting a maximum efficiency loss of 10%, i.e. a voltage drop of 70 mV from 0.7 V to 0.63 V over the total lifetime of the stack, the degradation rate for stationary systems should be less than 1.7 µV h−1, assuming 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours for the lifetime. For transport systems, where 5000 hours lifetime is taken for passenger cars, the maximum degradation rate should be less than 14 µV h−1. Due to the voltage loss, electrical efficiency will degrade and more heat will be released. In the case of combined heat and power (CHP) generation, the heat can be used. In transport applications, cooling problems have to be anticipated when degradation becomes too significant.
000 hours for the lifetime. For transport systems, where 5000 hours lifetime is taken for passenger cars, the maximum degradation rate should be less than 14 µV h−1. Due to the voltage loss, electrical efficiency will degrade and more heat will be released. In the case of combined heat and power (CHP) generation, the heat can be used. In transport applications, cooling problems have to be anticipated when degradation becomes too significant.
Both the electrodes as well as the proton conducting membrane are susceptible to ageing effects that will lead to performance loss of the PEMFC during its operating life. Performance loss can occur both in operation as well as when residing at rest.56 The proton-conducting polymer, present in the membrane as well as in the electrodes, can lose its conductivity by dehydration57 and by contamination with metal ions.41
The presence of ammonia in the fuel, which can be the case when operating on reformed fuels, has been shown to lead to irreversible performance loss.58 Ammonia reacts as a base with the acidic membrane, leading to a lowering of the proton conductance in the electrode.58 Pollutants in the air, notably NH3,59 SO259 and NO260 can lead to performance loss, both temporary and permanent.
Using pure hydrogen (99.9%) and oxygen (99.8%), PEMFC stack performance was monitored during an 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hour life test,61 in order to test the feasibility of the PEMFC stack for use in space shuttle applications. The degradation observed was very low, and amounted to a mere 16 mV at a current density of 0.86 A cm−2 over the total test time. The only component that showed some degradation was the sealant material. As relatively high noble metal loadings were used in the tested PEMFC stack, 4 mg cm−2 Pt at the cathode and 4 mg cm−2 Pt and 1.2 mg cm−2 Rh at the anode, the degradation associated with electrode poisoning might be much higher in PEMFC stacks with lower noble metal loadings. Also less pure hydrogen and ambient air will probably lead to higher degradation rates.
000 hour life test,61 in order to test the feasibility of the PEMFC stack for use in space shuttle applications. The degradation observed was very low, and amounted to a mere 16 mV at a current density of 0.86 A cm−2 over the total test time. The only component that showed some degradation was the sealant material. As relatively high noble metal loadings were used in the tested PEMFC stack, 4 mg cm−2 Pt at the cathode and 4 mg cm−2 Pt and 1.2 mg cm−2 Rh at the anode, the degradation associated with electrode poisoning might be much higher in PEMFC stacks with lower noble metal loadings. Also less pure hydrogen and ambient air will probably lead to higher degradation rates.
Use of fuel cells in transport applications means they should resist freezing conditions. Without precautions, freezing of the water contained in the membrane–electrode assembly leads to physical damage of the membrane–electrode interface.62 Effective removal of the water is possible by either gas purging36,62 or washing away with antifreeze liquids,62 preventing performance loss of the fuel cell. Membrane–electrode assemblies using reinforced membranes are much less affected by freeze–thaw cycles.63
Fuel starvation is another well-known cause of degradation of fuel cells.36 In a series of fuel cells, all cells are obliged to generate the same current. As long as all cells perform equally well and are supplied with enough fuel, all cells are operated at the same cell voltage and no degradation should occur. When however a single cell is not supplied with enough fuel, the cell materials are sacrificed to sustain the cell current. Cell materials to be oxidized are the carbon support of the catalyst36 and the bipolar plate material. Degradation can occur by fouling of the catalyst by their oxidation products, by increasing contact resistance or by complete loss of the electrode catalysts.36 Strategies to protect the fuel cell components from oxidation in case of fuel starvation focus on the promoting of water oxidation.36
Oxidant starvation leads to the recombination of protons to molecular hydrogen at the cathode.36 Although also in the case of oxidant starvation the cell voltage can drop below zero, physical damage is less severe than in the case of fuel starvation.
Pinholes in the electrolytic membrane are another well-known failure. They can be caused either by mechanical damage, or by local heat generation. Pinholes lead to direct mixing of hydrogen and air, which will react with formation of reaction heat leading to more cell damage.
PEMFC systems running on reformed fuels face, in addition to all durability issues addressed in the previous section, difficulties which stem from impurities present in the fuel reformate. Depending on the primary fuel, contaminants present in the reformate with negative effects on fuel cell lifetime are carbon monoxide,36 ammonia58 and sulfur containing components.60,64 The noble metals in the electrodes are easily poisoned by low concentrations of impurities. While the effect of CO and CO2 is reversible, sulfur components in the ppm range lead to irreversible loss of fuel cell performance, whether as part of the inlet air or fuel.59,63
As mentioned before, the negative impact of carbon monoxide is in many systems mitigated by using an air bleed. The catalytic reaction between CO and O2 can however lead to hot spots when the flow design of the fuel cell is not optimal.36
Most tests aiming at studying the PEMFC performance on reformed fuels are done using simulated reformate. While this covers the effect of operation on diluted hydrogen, CO2, and CO, effects of partly unknown impurities remain unaddressed.
For operation on simulated methane reformate, a degradation rate of 0.5 µV h−1 over 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours of operating time has been demonstrated.36 Osaka Gas has measured a degradation rate of 2 µV h−1 over more than 12
000 hours of operating time has been demonstrated.36 Osaka Gas has measured a degradation rate of 2 µV h−1 over more than 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours using simulated reformate gas.65 Operation of a 7400 hour field trial on real methane reformate has been completed successfully, without disclosing the degradation rate.36
000 hours using simulated reformate gas.65 Operation of a 7400 hour field trial on real methane reformate has been completed successfully, without disclosing the degradation rate.36
3. Current status of solid oxide fuel cells
The Solid Oxide Fuel Cell is a strong candidate for stationary power generation, especially in the power range of 1–200 kWe. Its high operating temperature allows operation on a wide range of fuels without the need for extensive reforming and gas clean-up steps as required in PEMFC systems. The cell reactions in the SOFC are depicted in Fig. 5. | ||
| Fig. 5 Basic principle of the SOFC, for the case where both hydrogen and CO are in the anode feed. | ||
Both the electrolyte as well as the electrodes consist predominantly of ceramic materials. Only the anode contains metallic nickel for electron transport and catalysis of hydrogen oxidation. The basic cell concept does not require noble metals. The electrolyte is a ceramic oxygen ion conductor.
Whereas the system of an SOFC is much less complex compared to the system of low temperature fuel cells, the major challenges are on cell and stack level.
Generally, SOFC developers consider three cell configurations: electrolyte-supported cells, anode-supported cells and metal-supported cells, depending on which component is the thickest and serves as the mechanical basis. In this order, these fuel cell configurations are also referred to as first generation, second generation and third generation. The temperature of operation decreases through these generations from 900–1050 °C for the electrolyte supported cell, to 700–800 °C for the anode supported and 500–700 °C for metal supported cells. Operation at lower temperatures is aimed at because of sealing issues and the need for using cheap, iron based heat resistant steels for separator plates (called interconnects in SOFC) and system components.
Due to the high operating temperature, an important factor in the design of a solid oxide fuel cell stack is the matching of thermal expansion of the cell components and interconnects, to prevent cracking of the intrinsically brittle ceramic cells, gas leakage and loss of electrical contact.
3.1. Electrolytes
The electrolyte that is generally used in the SOFC is yttria stabilised zirconia, abbreviated as YSZ, either ZrO2 doped with 3 mol% Y2O3 (3YSZ) or with 8% Y2O3 (8YSZ). The dopant concentration has a strong influence on ion conductivity and mechanical properties.66 In electrolyte-supported cells, the electrolyte is typically 120–150 µm thick. Because the conductivity is proportional to the temperature, operating at lower temperatures requires thinner electrolytes. At around 850 °C the electrolyte thickness must be so thin, that it cannot mechanically support the cell anymore. For operation between 600–800 °C, electrolyte layers are typically less than 20 µm.67An alternative for operation at lower temperature is the application of other electrolyte materials, for example CeO2 doped with 10 mol% GdO, abbreviated as GCO. Electron conduction that occurs at reducing conditions in the anode environment, leading to short-circuiting is an issue for this alternative electrolyte.67 For operation at 600 °C or even lower, La0.9Sr0.1Ga0.8Mg0.2O2.85 electrolytes offer superior conductivity, but exhibit stability problems caused by evaporation of Ga, and low mechanical stability and high gallium costs.67 Scandium doped ZrO2 offers improved oxygen ion conductivity and relatively high mechanical strength, at the expense of using high cost scandium.67
3.2. Electrodes
SOFC anodes are generally composed of Ni–YSZ. Besides catalysing H2 oxidation and facilitating electron conduction, nickel is active in reforming of carbon containing fuels, which is an attractive feature of the SOFC. As steam reforming of methane is an endothermic reaction, the heat produced at the anode can directly be used for the steam reforming reaction. Water formation at the anode side helps as well. A critical issue however is coke formation, which occurs at lower water contents. Direct injection of fuels such as ethanol and iso-octane has been shown to lead to immediate loss of SOFC performance caused by coke formation.68 Oxidation–reduction cycles can form another threat to anode stability. Anodes composed of nickel and gadolinium doped ceria appeared to have a much better resistance towards these oxidation–reduction cycles than Ni–YSZ anodes.69 Completely ceramic anodes, not containing nickel, show good oxidation–reduction cyclability.70 Another positive effect of replacing nickel is a better tolerance towards sulfur.70 However, the main challenge is to increase the electrochemical performance of the full-ceramic anode to the same level of the Ni based anode.SOFC cathodes consist of single-phase La0.75Sr0.2MnO3 (LSM) or of mixtures of this compound with YSZ. As in the other fuel cell types, the oxygen reduction largely determines the efficiency of the SOFC.67 Substitution of manganese with cobalt gives improved cathode performance.67 The mechanical properties of cobalt containing cells are however poorer, as the thermal expansion coefficient of the cobalt containing cathode is twice that of YSZ, and formation of low conductivity products at the electrolyte–cathode interface leads to decreasing power output.71 Cathodes using La0.6Sr0.4Co0.2Fe0.8O3 (LSCF) have the advantages of lower losses at lower temperatures (600–700 °C) and are additionally reported to be less sensitive to Cr-poisoning. As in other fuel cells, electrode optimisation is focused on improvement of the conductivity throughout the electrode for ions and electrons and the accessibility for oxidant.
Loss of cathode performance is associated with changes of microstructure and phase composition at load conditions. In combination with Cr containing steels for the interconnects, important degradation occurs due to poisoning of the cathode by Cr which evaporates from the steel and preferably condensates at catalytic sites. In general the rates of these degradation mechanisms decrease with decreasing operating temperature.72
In contrast to the PEMFC where electrolyte and electrodes can be manufactured separately and joined together at a later stage, solid oxide fuel cell manufacturing comprises electrolyte and electrode manufacturing in close conjunction.
Future SOFC cell cost needs to be less than US$500 m−2, leading to a cost of less than US$250 kWe−1 when operating at cell power densities of 0.2 W cm−2.73
Current price level estimates are US$2000–5000 kWe−1 for electrolyte-supported cells73 and US$12000 kWe−1 for anode-supported cells.73 Cheaper raw materials, simpler manufacturing procedures and above all mass manufacturing of cells are the keys to lower cell costs.73
3.3. Separator plates—interconnects
Separator plates are in SOFC mostly called interconnects. At high temperatures, one option is to use ceramic interconnects. The ceramic plates are based on LaCrO3. Doping with Ca, Sr or Mg leads to higher electrical conductivity.74 Pure ceramic plates have the tendency to be partially reduced at the anode side, leading to warping and breakage of the sealing.75 Besides, more cost effective materials and fabrication methods are needed for bringing this technology to the commercial stage.75 Metallic interconnects would lead to lower fabrication costs, are less brittle and have a higher electrical and thermal conductivity.74An extensive review on high temperature alloys and their suitability for application in the SOFC has been published recently.75 At temperatures below 800 °C, metallic interconnects, such as ferritic steels can be used. The advantages are lower costs and simpler manufacturing.72 Chromium containing alloys are used to ensure high temperature oxidation resistance and sufficient electron conductivity of the corrosion scale. Evaporation of this chromium, and its subsequent deposition at the cathode–electrolyte interface is one of the causes of SOFC degradation when using chromium-containing metallic interconnects.72 The application of contact coatings on the alloy can prevent the degradation caused by chromium evaporation and increase the electronic conductivity of the cell interconnect assembly.76 The typical requirements imposed by the SOFC conditions have led to a few new alloys, specially designed for application in the SOFC.77
3.4. SOFC—state-of-the-art performance
Table 3 summarizes the state-of-the-art performance of Solid Oxide Fuel Cells under different conditions. Planar cell power densities of 0.6–0.9 W cm−2 at a cell voltage of 0.7 V can be regarded as state-of-the-art for anode supported Solid Oxide Fuel Cells operated at temperatures of 750 °C and lower, at atmospheric pressure. Much higher power densities are reported at low fuel utilisation, typically 25%. Such low fuel utilisations are, from an efficiency point of view, not realistic for practical systems and therefore only data at fuel utilisation of 60% and higher are reported here.| Cell power density/W cm−2 at 0.7 Vcell | Cell type | Company/laboratory | Ref | |
|---|---|---|---|---|
| Global T. = Global Thermoelectric; PNNL = Pacific NorthWest National Laboratory; Siemens W. = Siemens Westinghouse.a Power density higher than 0.6 W cm−2 at fuel utilisation lower than 0.6. | ||||
| H2–air ambient pressure, 750 °C | 0.6a | ASC | Global T. | 78 |
| 0.9 | ASC | PNNL | 79 | |
| Methane + air ambient pressure, 720 °C | 0.55 | ASC | FZ Jülich | 80 |
| Simulated reformate + air ambient pressure, 750 °C | 0.44 | ASC | Delphi/Batelle | 81 |
| Natural gas + air ambient pressure, 850 °C | 0.1 | ESC planar | CFCL | 82 |
| Natural gas + air ambient pressure, 1000 °C | 0.14 | ESC tube | Siemens W. | 83 |
Electrolyte-supported cells give much lower power densities. Many stack and system developers still use electrolyte-supported cells as their robustness is better established.
Two SOFC stack configurations are in development: planar and tubular. The tubular SOFC is being developed by Siemens Westinghouse and Rolls-Royce. One of the main advantages of the tubular concept is the relative ease whereby the sealing between anode and cathode compartments can and has been solved. Thermal stress is a concern in the tubular design. As the tubular configuration is targeting large stationary applications with more or less continuous operation,84 this should not be a major barrier.
The power densities demonstrated with planar cells however are much higher, especially because current collection is much more effective than in the tubular cells.
It is generally believed that although the tubular SOFC is at present the most developed, in the long term planar SOFCs offer a better cost perspective and higher power densities.84
Power densities for SOFC stacks are not reported frequently. SOFC stacks are, besides their application in Auxiliary Power Units (APUs), primarily designed for stationary power generation and the focus in SOFC development is predominantly on increasing lifetime and robustness, more than on increasing the power density. Power densities as reported in Table 4, are on first sight considerably lower than for PEMFC stacks. When comparing the power density with the atmospheric PEMFC stack of 1.5 kWe, it must be concluded that both power densities are in the same range.
3.5 SOFC durability data
The durability of the SOFC is primarily determined by processes occurring during thermal cycles, oxidation–reduction cycles, more than accumulation of contaminants, as is the case for the PEMFC. Sulfur is an exception. Even at the high temperatures at which the SOFC is operated, sulfur is adsorbed by the anode and causes performance loss.88An important cause of degradation is loss of activity of the anode. Nickel sintering and coke formation when operated on carbon containing fuels lead to a loss of active surface area.72 Another factor which has been mentioned before is the deposition of chromium from the interconnect on the cathode–electrolyte interface.
An averaged degradation rate of 1% per 1000 hours over a total test period of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours has been reported by Global Thermoelectric for a single cell under realistic load, using hydrogen as fuel and at an operating temperature of 750 °C.89 A short stack operated on 50% hydrogen at 850 °C by Haldor Topsoe hardly suffered from any degradation90 during a 3000-hour operation period.
000 hours has been reported by Global Thermoelectric for a single cell under realistic load, using hydrogen as fuel and at an operating temperature of 750 °C.89 A short stack operated on 50% hydrogen at 850 °C by Haldor Topsoe hardly suffered from any degradation90 during a 3000-hour operation period.
Pressurised tubular stacks have been operated by Mitsubishi Heavy Industries for 7000 hours, the degradation rate is not reported.91
If the degradation rate is limited to 0.25% per 1000 hours, the power output can probably be maintained by increasing the stack temperature by 15 °C or lowering the fuel utilisation.90 For larger degradation rates, these solutions become less feasible.
For application in vehicles as Auxiliary Power Units, breaking of ceramic cells caused by vibrational forces is a real concern. Global Thermoelectric has shown that a 500 W stack can survive vibration of 10 G at 185 Hz.78
4. Hydrogen storage, transport and production
The Direct Methanol Fuel Cell is the only fuel cell in which a fuel other than hydrogen is electrochemically oxidized. In all other fuel cells, hydrogen or a hydrogen–carbon monoxide mixture (synthesis gas) is electrochemically oxidized. Hydrogen can be either generated internally, as is done in the MCFC and in large fraction of the SOFC stacks, or be supplied externally. As displayed in Fig. 4, the hydrogen can be supplied as pure hydrogen, or generated within the system in a so-called fuel processor or reformer.Fig. 6 gives an overview of a variety of the most common fuel supply chains in combination with the fuel cell types.
For obtaining hydrogen from solar and wind power, electrolysers are commonly used. Electrolyser technology is not covered in this review. Electrolyser efficiencies of commercial alkaline electrolysers are in the 65–75% range.92
For the generation of hydrogen from fossil fuels as well as biofeedstocks, thermal conversion processes are used, either as the central production unit, as a decentral unit or as part of the fuel cell system. For stationary applications, natural gas will be the preferred fuel in the coming decades, as supplies are sufficient and existing distribution networks can be used. For vehicles, pure hydrogen is considered as one of the options. In that case, distribution networks as well as storage need to be available.
4.1. Hydrogen storage and transport
Several options are in development: liquid hydrogen, pressurized hydrogen, metal hydrides, borohydrides and storage in carbon structures.
State-of-the-art compressed hydrogen storage consists of lightweight tanks using polymers and carbon fibers containing hydrogen compressed to 700 bar. Liquid hydrogen, stored at −253 °C, is stored in tanks that are engineered in such a way that boil-off losses are minimised.96 It strongly depends on the driving behaviour whether boil-off losses are acceptable or not.95 The workday driver with a minimum driving range of 25 km per day would not suffer from loss of fuel, while the weekend driver driving 50 km per day would suffer from 15% loss of fuel.95
To avoid the energetic losses associated with compression and liquefaction of hydrogen (see next section), metal hydrides have been under investigation for quite some time. Lightweight elements are under special consideration, to meet the weight target of the storage vessel. Mg, LiN and NaAlH4 are lightweight candidates, but suffer from the high temperatures, 200–300 °C, at which desorption takes place.97
Hydrogen storage in carbon nanotubes has up to this moment not fulfilled initial expectations.98 Zeolites are under consideration as hydrogen storage materials as well.97,98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2, approaching a refueling density of 1 per 10 km2.The USA has 187
000 km2, approaching a refueling density of 1 per 10 km2.The USA has 187![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 petrol stations, Western Europe 80
000 petrol stations, Western Europe 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
An infrastructure consisting of hydrogen fuel stations will cost approximately 10 times that for new liquid fuels as methanol or ethanol.100 Using existing gasoline and diesel infrastructure would impose no extra infrastructural cost. For safety reasons, a hydrogen filling station will be quite different from the gasoline stations, as we know today.
Also from an energetic point of view, large-scale transportation of hydrogen, and the necessary compression or liquefaction of hydrogen can be highly unattractive.101 Both compression (10–20%)97,101 and liquefaction (25%–40%)97,101 consume an unacceptable part of the energy content of the hydrogen. New methods of liquefaction, such as magnetic and acoustic refrigeration, could diminish the energy use for liquefaction.97 In addition, transport by trucks or through pipelines over large distances should be avoided: hydrogen trucks consume 20% of the energy content of the hydrogen transported per 100 km delivery distance, pipeline transport consumes 10% of the hydrogen energy content per 1000 km.101
Production of hydrogen at the “petrol” station would avoid the efficiency losses associated with transport of hydrogen.
4.2. Fuel processor technology
On site generation of hydrogen can speed up the introduction of fuel cell systems without the presence of a widespread hydrogen infrastructure. The generation of hydrogen from hydrocarbons is a multi-step process, which is schematically displayed in Fig. 7.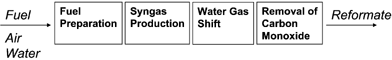 | ||
| Fig. 7 Schematic overview of hydrogen generation by means of fuel processing. | ||
The purpose of the fuel processing is to generate a reformate which is most suitable for the fuel cell in question. The tolerance towards carbon monoxide strongly depends on the temperature level of the fuel cell. The MCFC and SOFC can be fed with carbon monoxide, while the concentration of CO that can be tolerated by the PEMFC is in the range between 10–50 ppm. Other impurities with a negative impact on fuel cell performance and durability have to be removed as well.
Gasoline fuel processors are quite scarce. A partially integrated gasoline fuel processor for a 10 kWe PEMFC stack, consisting of an autothermal reformer, a desulfuriser and a single stage shift reactor, was demonstrated by Argonne National Laboratory. The volume of this system, which needs an additional PrOX reactor, amounts to 7 l.103
Both Nuvera as well as Hydrogen Source (A Shell/UTC Fuel Cells joint venture, liquidated mid 2004) have developed a gasoline fuel processor, which is suitable for use in passenger vehicles. The Hydrogen Source gasoline fuel processor has a cold start-up time of 4 minutes, which is relatively short, but still too long to meet the DoE target of 30 seconds.
The Nuvera Star gasoline fuel processor,104 which can be operated on ethanol and natural gas as well, has an efficiency of 80% and can generate the hydrogen for a 62 kWe fuel cell system. The CO concentration in the reformate amounts to 50 ppm, while the volume of the system amounts to 75 l.
Daimler Chrysler concluded on the basis of simulations that while methanol fuel processors can be highly integrated, leading to a compact fuel processor, gasoline fuel processors couldn't be integrated far enough, due to too large temperature differences between the several stages.100 Both dynamics and efficiency would be poor to compete successfully with e.g. diesel internal combustion engines.100
Based on the current status of fuel processors for transport, as displayed in Table 6, the DoE has decided mid 2004 to terminate the funding of the development of gasoline on-board fuel processors for vehicle propulsion.
| Power density/kWe l−1 | Efficiency (%) | Start-up energy/MJ (50 kWe)−1 | Durability/h | Start-up time/s | Cost/$ kWe−1 | |
|---|---|---|---|---|---|---|
| DoE 2004 target | 0.7 | 78 | <2 | 2000 | <60 | — |
| DoE 2010 target | 2 | 80 | <2 | 5000 | <30 | <10 |
| Status 2004 | 0.7 | 78 | 7 | 1000 | 600 | <65 |
Important factors in the decision of the DoE are:
– the progress made with hybrid ICE vehicles with respect to fuel economy
– the expectation that the extra effort put into supporting a hydrogen based transport system by the Hydrogen Fuel Initiative of the Bush administration, will shorten the time a transition technology such as gasoline–fuel cell vehicles will be used.105
– automotive manufacturers do not show much interest in the option of on-board fuel processing anymore.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours, and lifetime is expected to be more than 90
000 hours, and lifetime is expected to be more than 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours. The high hydrogen concentration is a major advantage of steam reforming. The start-up time is 1 hour, which is relatively long for residential applications. The volume of the complete fuel processor amounts to 48 l, including thermal insulation.
000 hours. The high hydrogen concentration is a major advantage of steam reforming. The start-up time is 1 hour, which is relatively long for residential applications. The volume of the complete fuel processor amounts to 48 l, including thermal insulation.
Similar fuel processors have been developed by Tokyo Gas,107 Nuvera,108 Plug Power, Johnson Matthey and many others for natural gas or, amongst others, by Sanyo for propane.109
Shell/Hydrogen Source developed a 2 and 5 kWe integrated fuel processor for natural gas and propane based on catalytic partial oxidation.
5. Fuel cell systems and field trials
Fuel cell field trials play an extremely important role in the improvement of fuel cells and their introduction into the market. Both in transport as well as in stationary markets, fuel cells replace technologies which have been on the market for more than a century, meet customer requirements satisfactorily and have evolved into a low cost commodity through many years of strong, global competition.Fuel cells will not be allowed to develop through the development curve in the market as e.g. cars were allowed during their first decades. The price![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) quality ratio that cars have displayed in their first decades, will be totally unacceptable in the present market for the vast majority of consumers, bar a small number of early adapters. A new technology needs to be better than the technology it replaces. Environmental benefits are not enough to convince consumers to switch to a new technology.
quality ratio that cars have displayed in their first decades, will be totally unacceptable in the present market for the vast majority of consumers, bar a small number of early adapters. A new technology needs to be better than the technology it replaces. Environmental benefits are not enough to convince consumers to switch to a new technology.
The goals of field trials are to evaluate the technology through a wide range of conditions, to show the public the capabilities of the new technology, and not unimportantly, to increase production numbers and thereby reduce cost through economies of scale.110
Early market introduction and demonstration is seen mostly in stationary off-grid applications or as back-up power in critical environments, where operating hours are low and existing technologies, such as diesel generators, have serious disadvantages. The efficiency, which is at present still too low for many current systems, is not a key factor in these markets. Other niche applications for fuel cells are auxiliary power units in cars and leisure applications, backup power systems in offices and houses, replacing diesel generators. Applications for the military range from power packs for soldiers to MW systems in submarines.
5.1 Stationary applications of PEMFC and SOFC
For decentralised power generation, fuel cell systems are being developed which run mostly on natural gas, sometimes on propane or even kerosene in the case of Japan.111 Inside the systems, hydrogen is generated by steam reformer or autothermal reformer based fuel processors.One of the frontrunners in the demonstration of PEMFC systems for stationary applications is Plug Power. Systems of 5 kWe, operated on either natural gas or propane commercialised under the trade name GenSys, are being demonstrated at the United States Military Academy and other sites by the Department of Defense.112,113 The Plug Power CHP systems are operated on natural gas and produce at maximum power 5 kW electric and 9 kW thermal. The electrical efficiency of these GenSys systems amounts to 24.8%, which is an average of different operating set points. NOx and SOx emission concentrations are below 1 ppm. The average availability has been improved from 88% in year 2002/2003 to 92% in year 2003/2004.
In Europe, 31 Plug Power systems are being evaluated within an EU funded project called the Virtual Power Plant. All systems will be grid connected and centrally controlled, in such a way that together these systems form a virtual power plant.99
Japanese industries and gas utility companies are very actively developing small micro CHP systems: Hitachi,114 Tokyo Gas,115 Fuji Electric,116 Osaka Gas64 and many more. In a Japanese program, called the Millennium Project, micro combined heat and power systems are being evaluated.117 The system size is typically between 1 and 5 kWe (Fig. 8). Participating companies are Toshiba IFC, Sanyo Electric, Toyota, Plug Power, Mitsubishi Electric, Ebara Ballard, Matsushita Electric and UTC Fuel Cells. The electrical efficiency of the Japanese 1 kWe systems is typically 30%.
 | ||
| Fig. 8 1–5 kWe Residential fuel cell systems under evaluation in the Japanese Millennium Project.117 | ||
The systems that are available now should be seen as the first generation, suitable for field trials but not for large-scale market penetration. The necessary reliability and lifetime have not been demonstrated. Besides that, electric efficiency needs to be improved to at least 35%. With respect to the cost level, it is expected that at large volumes the cost target of $1000–1500 kWe−1 can be met when using the materials available at this moment.
PEMFC stationary systems of 250 kWe have been developed by an Alstom–Ballard joint venture.118 Five plants with an electrical efficiency of 34% and a total efficiency of 73% have been tested in field trials since 2000.118 Further commercialisation plans for the 250 kW systems are unclear.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours at an AC efficiency of 46%, without any voltage degradation.120 Bigger units, of 170 kWe and 190 kWe have been put in operation since, but not for as long as the 110 kWe system.
000 hours at an AC efficiency of 46%, without any voltage degradation.120 Bigger units, of 170 kWe and 190 kWe have been put in operation since, but not for as long as the 110 kWe system.
Derived from this concept and in collaboration with Siemens Westinghouse, 5 kWe tubular systems have been put in operation by Fuel Cell Technologies. The AC efficiency of these smaller systems is reported to amount to 38%120 and they have been in operation for more than 1700 hours.
Sulzer Hexis has concluded a field test with its Hexis 1000 Premiere systems, a 1 kWe system that with an additional burner covers the full heat demand and the base load electrical demand of a single family house. The AC efficiency of this system amounts to 25–32% at full load.121,122 For commercial introduction, this generation has shown a too high degradation rate. In addition further reduction in size, weight and cost are needed. A redesign consists, amongst other things, of changing from natural gas steam reforming to catalytic partial oxidation of natural gas and a new design of the metallic interconnect.121
A 2 kWe system from Global Thermoelectric running on natural gas has been operated for 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours at a maximum AC efficiency of 29%.123 Better thermal integration and higher fuel utilisation, 60–70%, in the next generation should lead to an increase in efficiency to 35%.123
000 hours at a maximum AC efficiency of 29%.123 Better thermal integration and higher fuel utilisation, 60–70%, in the next generation should lead to an increase in efficiency to 35%.123
A pressurised (4 bar g) 10 kWe SOFC module of Mitsubishi Heavy Industries, consisting of 288 SOFC tubes with internal natural gas reformer has been operated with a DC efficiency of 41.5% HHV for 755 hours.124 A former generation has been operated for 7000 hours.
A consortium of Wärtsilä and Haldor Topsoe is developing a 250 kWe SOFC system, based on planar SOFC technology. Based on laboratory experiments and detailed engineering calculations the consortium expects that 250 kWe plants can become competitive to 300 kWe gas engine plants between 2010 and 2020.125 Total unit price has been calculated to be in the range of 1600–2600 € kWe−1 in 2010 and 676–1100 € kWe−1 in 2020.125 Whereas SOFC stacks contribute 310 € kWe−1, balance of plant costs would contribute as much as 490 € kWe−1 to this 2020 cost estimate.125
For auxiliary power units, BMW/Delphi is the leading consortium. The ongoing electrification of vehicles is hitting the boundaries of conventional batteries and generators. This has lead to the insight that an auxiliary power unit (APU), which consists of a fuel cell system decoupled from the drive train, can generate the power needed on board both when driving as well as during standstill. As the APU might be introduced before fuel cell systems are ready for introduction in the drive train of the vehicle, regularly used fuels such as gasoline and diesel are the fuels of choice. As the available space in existing vehicles for an additional device is limited, SOFC systems are seriously considered by the automotive sector for APU's. For the SOFC, fuel processing of gasoline and diesel will be much less complex than in the case of a PEMFC. Compared to stationary applications, the APU puts more challenging demands on the SOFC with respect to the power density, the start-up time and thermal cycling capability. A gasoline SOFC APU system has been demonstrated by Delphi/BMW integrated in a BMW vehicle.79 Their latest generation APU has a start-up time of 60 minutes.126
5.2 Application of PEMFC systems for transport
A considerable number of fuel cell vehicles are presently being tested and demonstrated on the road. These tests show the advancement of the fuel cell technology with respect to robustness, compactness and driving performance. It does not give the progress with respect to cost reduction. Table 7 gives an overview of part of the fuel cell vehicle demonstrations.| Company | Vehicle type | Type | Fuel | Year | Accomplishment |
|---|---|---|---|---|---|
| Daimler Chrysler | Small passenger car | Necar 5 | Methanol | 2002 | USA coast to coast trip, 4500 km130 |
| Volkswagen | Mid size passenger car | Bora HY Power | Hydrogen | 2002 | Mid winter mountain trip across Simplon Pass (CH) at −9 °C |
| General Motors | Mid size passenger car | HydroGen3 | Hydrogen | 2004 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km Journey in 38 days 000 km Journey in 38 days |
| Daimler Chrysler | Small passenger car | Mercedes A F-cell | Hydrogen | 2004 | 60 Cars in operation in Germany, Japan, USA and Singapore102 |
| Daimler Chrysler | Bus | Citaro | Hydrogen | 2003 | 30 Buses in 10 European cities in daily operation102 |
Daimler Chrysler has been the pioneering car manufacturer since the mid 1990s. Through various generations, system size has diminished tremendously in close cooperation with Ballard. Initially, the fuel cell system was so large that only a minivan could accommodate it (Necar 1 and Necar 2).101,102 In the newest model, the fuel cell system is situated in the floor space of a Mercedes A-class passenger vehicle, hardly sacrificing the customer need for space.102 At present, all major car manufacturers have a development program for fuel cell vehicles. The most active manufacturers are, besides Daimler Chrysler: Toyota, Ford, General Motors and Honda. A complete, actualised overview is available at the website of FuelCells2000.127
The majority of the vehicles run on hydrogen. Daimler Chrysler, Toyota and General Motors have demonstrated vehicles, which produced hydrogen on board using fuel processors, mostly running on methanol or specially formulated gasoline type of fuels. At present, most manufacturers are focusing on further development of vehicles with on-board hydrogen storage.
Field trial programs, in which fuel cell vehicles are tested in realistic environments, are running in California in the California Fuel Cell Partnership128 and in Japan within the Fuel Cell Commercialisation Conference of Japan.
Vehicle systems often combine fuel cells with an electricity storage package, which can be either batteries or super capacitors.129 Three important reasons for using electricity storage devices in fuel cell vehicles are: improvement of dynamics, decreasing fuel cell stack size and cost, and enabling regenerative braking, which has a positive impact on the total efficiency.
Fuel cell buses have been in development since the 1990s. The advantages of fuel cells in buses are multiple. From a technical point of view, the ample availability of space has made it easy to integrate the system and hydrogen storage in the bus without sacrificing the available space for passengers. Availability of technicians at bus terminals makes a field trial easier to handle, and fuelling of buses is generally done at a central depot. Finally, the dynamics of a bus drive cycle is especially advantageous for a fuel cell system in comparison to a diesel engine. The engine is often operated at partial load, leading to poor diesel engine efficiency. A Scania passenger bus, consisting of a 50 kWe hydrogen fed PEMFC system combined with a 135 kW battery system was tested on Braunschweig and FTP-75 duty cycles. Fuel consumption in the vehicle is 42–48% lower than in its standard diesel ICE version.53 The regenerative braking, which can also be applied in combination with an ICE hybrid vehicle, accounts for roughly half of the fuel saving.
The largest field trial of fuel cell buses is at this moment running in Europe in the EU funded CUTE project, where in 10 cities 30 Daimler Chrysler buses are in daily operation (Fig. 9).
 | ||
| Fig. 9 Fuel cell bus in Amsterdam in daily operation, as part of the EU-CUTE project. Photo by René van den Burg. | ||
The Department of Energy of the USA government has set technical as well as cost targets for mobile fuel cell systems which have to be met in order to become competitive with conventional cars.93 The direct hydrogen fuel cell power system has to have a 60% electric efficiency at a cost of $45 kW−1 by 2010 and $30 kW−1 by 2015, both including hydrogen storage. Alternatively, a reformer based fuel cell power system, operating on clean hydrocarbon or alcohol that meets emission standards, has to have a 45% electric efficiency at a cost of $45 kW−1 by 2010 and $30 kW−1 by 2015. The start-up time of a reformer based system should be less than 30 seconds.
The price of today's demonstration vehicles, $1 million for GM's HydroGen3 vehicle,131 stands in no relation with the vehicle price when manufactured in series. The cost of fuel cell systems for mobile applications is estimated to be at present at a level of $325 kWe−1,130 at a production level of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per year. According to the DoE 2003 Progress Report, the current cost level, 2003, is $250 kW−1 at a volume of 500
000 units per year. According to the DoE 2003 Progress Report, the current cost level, 2003, is $250 kW−1 at a volume of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per year.
000 units per year.
5.3 Applications of other fuel cell types
The major advantage of the alkaline electrolyte is the possibility to use non-noble metal catalysts for both the anode as well as the cathode. For the anode, nickel133 can be used, while silver can be used for the cathode.134 Both alternatives do however suffer from degradation.133,134
Due to its intolerance to CO2, both as a component in the fuel as well as in the air, its practical use for mobile applications as well as stationary power generation is rather limited. The reaction between CO2 and KOH leads to precipitation of K2CO3, due to its limited solubility at low temperatures. This precipitated K2CO3 blocks the porous electrode structures, especially when using Raney nickel mesh electrodes. Filtering the CO2 out of the cathode stream (as well as the anode stream) is possible using a limestone filter. This would imply a usage of limestone of 0.1–0.01 kg per kWh of generated electricity.132 Another way to deal with CO2 is by circulating the electrolyte such that the CO2 and carbonate do not exclusively build up in the electrodes.134 The role of CO2 in the degradation of the AFC was recently shown to be minor, in comparison with the loss of the hydrophobic nature of the PTFE in the electrodes.135
The corrosive nature of hot KOH limits the choice of materials. Current collectors, seals and non-noble electrode catalysts are attacked by the KOH, even PTFE which is part of the electrode, suffers from degradation by KOH in combination with radicals formed by partial reduction of oxygen.134,135 Alkaline fuel cells with immobilised KOH suffer more from degradation than AFC’s with circulating electrolyte, and more in open circuit conditions than under load conditions.136 Carbon corrosion at high voltage in open circuit and carbonate build-up are responsible for this degradation.136
Cell power density of the AFC on hydrogen–air, at atmospheric pressure and 75 °C is in the range of 0.1–0.3 W cm−2.132 Pressurised systems are generally applied in space applications. In this application, oxygen is used as the oxidant, and power densities can be as high as 0.74 W cm−2.132
The alkaline fuel cell has been used in the majority of the space missions as power generator and potable water source. The AFC is also in development for small stationary power generation in the kW range. The limited lifetime of the AFC, being not more than 5000 hours, is a major hurdle for large-scale commercialization.134
Early transport applications used alkaline fuel cells as well. At present, none of the car manufacturers take AFC's into consideration. The cost of an atmospheric alkaline fuel cell system has been calculated to amount to $200–1750 kW−1, dominated by the stack costs.132
The effort put into the development of the molten carbonate fuel cell has been declining since the end of the 1990s. MCFC have been developed for stationary applications of 200 kWe and more. It can be operated on natural gas, sewage gas, and biogas.
The main industrial developers of MCFC units are Fuel Cell Energy and MTU, a subsidiary of Daimler Chrysler. MTU is putting 200 kW so-called HotModules on the market, primarily as demonstration units. The HotModule is operated at ambient pressure and uses internal reforming. Fuel Cell Energy has built a 2 MW plant in California.137
Chubu Electric Power Company and Toyota in Japan have both established 300 kWe MCFC units. The Chubu Electric unit is operated on digester gas, the Toyota unit is combined with a gas turbine.
Estimated price level given by MTU amounts to €1300–1500 kWe−1.138 HotModule plants have been tested in at least three field trials since 1999 in Germany.139 Fuel cell stack efficiency of 52% is reported.139
In fact, in the early stages of the PEMFC many components of the PAFC were adopted by the PEMFC. Only later, PEMFC specific optimisations were made which led to rapid improvement of the PEMFC. Due to the operating temperature which is more than 100 °C higher than the PEMFC, the tolerance towards carbon monoxide is much higher, typically 1–2%. Also the heat management is simpler in the case of the PAFC, and the quality of the heat is higher.
The phosphoric acid fuel cell is the fuel cell which has dominated the stationary market in the 1990s, with a (demonstration) market share of more than 80%.11 The PAFC systems are generally in the power range of 50–200 kW.
In recent years, its share has declined, as competing technologies are believed to be more cost effective in the long run. The PAFC can be regarded as being at the end of its development stage, and to have hit the bottom of its cost lowering asymptote. The installation cost of 200–1000 kWe systems are in the range of $2000–$4000 kWe−1,142,143 which is considerably higher than the $1000 kWe−1 which is generally believed to be required to be competitive for stationary applications.144
The cell power density of the PAFC is 0.14 W cm−2 when operated on hydrogen and air at atmospheric pressure.141,145
Main industrial PAFC developers are UTC Fuel Cells, Fuji, Mitsubishi and Toshiba.
A fleet of 30 PAFC systems of 200 kW electric power, manufactured by ONSI/UTC Fuel Cells under the trade name PC25, has been operated by the Department of Defense from 1997 till 2003 throughout the US at different climate conditions ranging from Alaska to Texas. Most units have been in operation for 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours, at an average availability of 66%.146 The averaged electric efficiency of the units amounted to 31.6%. Desert operation leading to water management troubles and operation in cold sites leading to freezing damage led to retrofits and redesigns, after which the performance and availability improved.110
000 hours, at an average availability of 66%.146 The averaged electric efficiency of the units amounted to 31.6%. Desert operation leading to water management troubles and operation in cold sites leading to freezing damage led to retrofits and redesigns, after which the performance and availability improved.110
Degradation rates of the PAFC stacks amounts to 5% per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours for the improved versions. Electrolyte depletion is the major cause of stack degradation.110 Emission levels of NOx, CO and VOC's and SOx were below 1 ppm, 5 ppm, 1 ppm and the detection limit of SOx respectively.110
000 hours for the improved versions. Electrolyte depletion is the major cause of stack degradation.110 Emission levels of NOx, CO and VOC's and SOx were below 1 ppm, 5 ppm, 1 ppm and the detection limit of SOx respectively.110
Also in Japan, PAFC systems have been operated for more than 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours, using UTCFC PC25 systems as well as Fuji FP100 systems.147 A number of PAFC systems are operated on digester gas instead of natural gas.
000 hours, using UTCFC PC25 systems as well as Fuji FP100 systems.147 A number of PAFC systems are operated on digester gas instead of natural gas.
6. Environmental benefit of fuel cells
6.1. Fuel cells for transport
The main drivers for fuel cell vehicles are to diminish the polluting emissions and surpass the poor efficiency of conventional transport, to become less dependent on foreign oil and to prepare the society for the after-oil era.| CO | NMHC | NOx | PM | |
|---|---|---|---|---|
NMHC = non-methane hydrocarbons; PM = particulate matter.a Emission standards for first 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 miles of vehicle life cycle. 000 miles of vehicle life cycle. |
||||
| Euro IV gasoline (2005) | 1 | 0.10 | 0.08 | — |
| Euro IV diesel (2005) | 0.50 | — | 0.25 | 0.025 |
| USA Tier 2 (2007) | 2.6 | 0.056 | 0.056 | 0.0062 |
The USA Tier 2 standard is an average standard which has to be met by a car manufacturer for his whole passenger car fleet. The Tier 2 standard is subdivided into eight so-called Bins, of which the Bin in Table 8 is the average Bin. In contrast to the Euro emission standard, under the USA Tier 2 legislation gasoline and diesel passenger cars have to meet the same emission standards.
A recent study by General Motors and LBST150 compares a wide variety of fuel pathways and powertrain systems, with respect to their energy use and CO2 emissions. Table 9 compares greenhouse gas emissions for a variety of fuel pathways.150 From Table 9 it follows that hydrogen production by electrolysis using electricity from the grid should be avoided, as it leads to the highest CO2 emission. Production of compressed hydrogen is to be preferred in comparison to liquid hydrogen.
| Fuel | Emission of CO2 eq/g MJLHV−1 | Supply cost/$ GJ−1 (cost of fuel, production, transport and refueling) |
|---|---|---|
| a CO2 eq includes emissions of CH4 (=21 × CO2) and N2O (=310 × CO2). b Price includes CO2 storage. | ||
| Gasoline | 13 | 8–10 |
| Natural gas | 14 | 7–9 |
| Liquid hydrogen from NG | 124 | |
| Compressed hydrogen from NG | 103 | 12–18b |
| Compressed hydrogen from EU-mix electricity | 208 | |
| Compressed hydrogen from biomass (poplar plantation) | 22 | 14–25 |
| Compressed hydrogen from wind via electrolysis; highest cost for off-shore | 0 | 22–37 |
When the choice of the fuel pathway is left to the market, the supply cost will be more important than the CO2 emissions for the various supply chains. Hydrogen produced by solar PV via electrolysis leads to a cost of $52–82 GJ−1.146
Fuel consumption as modeled in the GM study for various configurations is given in Table 10.
| Fuel consumption/l gasoline eq. (100 km)−1 | CO2 eqa emission, well-to-wheel/g km−1 | |
|---|---|---|
| a CO2 eq includes emissions of CH4 (=21 × CO2) and N2O (=310 × CO2). | ||
| 2002 Gasoline ICE car | 8.15 | 224 |
| 2010 Gasoline ICE car | 7.66 | 211 |
| 2010 Gasoline ICE hybrid vehicle | 5.61 | 154 |
| 2010 Diesel hybrid ICE vehicle | 5.18 | 137 |
| 2010 Fuel cell hybrid vehicle with on-board fuel processor on gasoline | 4.84 | 133 |
| 2010 Fuel cell hybrid vehicle on compressed hydrogen from NG | 3.31 | 108 |
| 2010 Fuel cell hybrid vehicle on compressed hydrogen from biomass | 3.31 | 23 |
| 2010 Hydrogen ICE car, compressed hydrogen from NG | 6.37 | 209 |
| 2010 Hydrogen hybrid ICE car | 4.68 | 153 |
A clear conclusion from Table 10 is that only hydrogen fuel cell vehicles offer a clear benefit with respect to a reduction of greenhouse gas emissions. Fuel cell vehicles with gasoline fuel processors do not provide such a benefit, in comparison to a diesel hybrid vehicle, neither do vehicles using hydrogen in internal combustion engines.
The data from Table 10 are in line with a recent study from MIT.151 Fuel cell vehicles (hybrid and non-hybrid) using gasoline will not have a significantly lower energy consumption and greenhouse gas emission than a hybrid internal combustion engine running on diesel. As the authors state themselves, fuel cell vehicles will be superior with respect to the emissions of non-greenhouse gases, such as NOx, SO2, hydrocarbons, CO and particles. In addition, it should be recognised that if hydrogen replaces gasoline and diesel for other reasons, then fuel cells will convert hydrogen much more efficiently than internal combustion engines.
Whereas well-to-wheel studies compare the same vehicle types with respect to weight, power to weight ratio and other vehicle specific characteristics by model calculations using the same driving cycles, comparison of existing vehicles under these equal circumstances proved unavailable from open sources. Table 11 gives an overview of CO2, NOx, CO and hydrocarbon (HC) emissions from sets of comparable existing vehicles. The Toyota Prius HSD is based on the same chassis as the Toyota Avensis. The Mercedes A vehicles are based on the same model as the Necar 5. The shortcoming of such a comparison is the difference in driving cycle (Japan 15 cycle for both fuel cell vehicles, EU drive cycle used in Cleaner Drive, for the other vehicles) and the difference in vehicle weight. It gives nevertheless an insight into the present state of the technology, as well as an idea of the results of well-to-wheel modeling.
| Toyota Avensis gasoline152 | Toyota Prius HSD gasoline152 | Mercedes A gasoline152 | Mercedes A diesel152 | Daimler Chrysler Necar 5 methanol102,150 | Toyota FCHV-4 hydrogena153 | |
|---|---|---|---|---|---|---|
| ttw = Tank-to-wheel; wtw = well-to-wheel; — = no data available.a Produced by steam reforming of natural gas.b Including CO2 emissions for fuel production, transport and distribution as given in GM study. Fuel Cell vehicle emissions as measured in Japan Drive Cycle. | ||||||
| Weight/kg | 1275 | 1400 | 1040 | 1085 | 1430 | 1860 |
| Power/kW | 95 | 57 | 75 | 70 | 75 | 80 |
| CO2 (ttw/wtwb) | 171/202 | 104/123 | 172/212 | 139/158 | — | 0/80 |
| CO ttw | 0.480 | 0.180 | 0.202 | 0.407 | 0.008 | 0 |
| NOx ttw | 0.050 | 0.010 | 0.024 | 0.381 | 0.000 | 0 |
| HC ttw | 0.030 | 0.020 | 0.054 | 0.000 | 0.036 | 0 |
| PM ttw | 0.000 | — | 0.000 | 0.039 | — | 0 |
The low contribution of ICE hybrid vehicles on reduction of non-greenhouse gases, as shown in Table 11, is confirmed in Toyota's Prius Green report, which evaluates emissions of CO2, NOx, HC, SO2 and particles over the entire life cycle of Toyota's newest gasoline ICE hybrid vehicle, the new Prius HSD, in comparison with a gasoline car of comparable size.154 It appears that besides the 35% reduction in CO2, the reductions of NOx, HC, SO2 and particles are respectively 8%, 16%, 4% and −50% (i.e. particulate matter emissions are higher for the hybrid vehicle than for the gasoline vehicle). The exhaust emissions of NOx and HC in the driving cycle, as measured in g km−1, are equal for both vehicle types.154
The emissions of fuel cell vehicles depend on the fuel being used. Tailpipe emissions from hydrogen-fueled vehicles are zero. The Daimler Chrysler Necar 5 is the fuel cell equivalent of the Mercedes A passenger car and runs on methanol. All tailpipe emissions of the Necar 5 vehicle are lower than its ICE equivalent.102,152 Comparison of Table 11 with Table 8 shows that while modern conventional gasoline vehicles will be able to meet Euro IV and Tier 2 emission standards, even small passenger diesel vehicles will have difficulties in meeting both Euro IV as well as USA Tier 2 standards. An impressive effort is being made to meet todays and future emission standards for diesel cars, by the development of e.g. NOx absorbers, hydrocarbon adsorbers, and combinations of regenerable particle filters and NOx traps. Through the introduction of advanced particle filters and NOx absorbers a large reduction potential is present, although the regeneration of soot filters and the NOx absorbers will lead to a considerable fuel penalty.155 Fuel cell technology offers the potential to reduce the non-greenhouse gas emissions while at the same time reducing the fuel consumption of the vehicle.
6.2. Decentralised power generation
The generation of electricity and heat at the site of demand can save a significant amount of primary energy compared to the central generation of electricity and the generation of heat on site. Besides dumping the waste heat generated at central production, electricity is lost during transmission and distribution, ranging from more than 6% in the EU15 countries and North America, to more than 10% in developing countries.146Both PEMFC and SOFC systems are in development for this combined heat and power generation on the household scale (1–5 kWe). As presented in the previous sections, large scale CHP, PAFC and MCFC systems are available, while SOFC and PEMFC systems are in development. Depending on the consumer price of natural gas and the consumer price of electricity, the ratio of which can vary significantly from country to country, such systems can be operated economically. It was calculated that in Germany, taking into account the heat and electricity demand of the houses and the price of electricity and natural gas and the fee received for electricity sold back to the distribution companies, the penetration of fuel cell systems could be in the order of 30% of natural gas supplied houses and 50% of heating oil supplied houses.156 An investment cost of less than €1000 kWe−1 was assumed in this calculation for natural gas systems and around €900–1200 kWe−1 for heating oil systems.
For stationary applications, the Department of Energy of the USA government has set the target for fuel cell systems operating on natural gas or propane being 40% electrical efficiency, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours durability at a cost of $400–$750 kW−1.
000 hours durability at a cost of $400–$750 kW−1.
In a way similar to well-to-wheel studies, emissions have been calculated for the full fuel chain for stationary applications. For the UK, the environmental impact of combined heat and power systems on a 200 kWe scale has been calculated using diesel engines, gas engines and fuel cell systems in comparison with central electricity production using Combined Cycle Gas Turbines (CCGT) and decentralised heat production using heating boilers.157Table 12 gives the energy input and emissions per kWh energy demand for various technologies compared in this study.
| Energy input/MJ kWh−1 | CO2/g kWh−1 | CH4/g kWh−1 | NOx/g kWh−1 | SOx/g kWh−1 | CO/g kWh−1 | |
|---|---|---|---|---|---|---|
| Grid electricity from NG (CCGT) + NG heating boiler | 5.65 | 270 | 0.194 | 0.310 | 0.007 | 0.141 |
| Diesel CHP engine | 4.75 | 315 | 0.08 | 4.432 | 0.685 | 0.222 |
| NG CHP engine | 4.40 | 219 | 0.311 | 1.246 | 0.006 | 0.996 |
| NG-SOFC | 4.40 | 219 | 0.150 | 0.021 | 0.005 | 0.001 |
Whereas the largest saving of energy originates from the combination of heat and power generation, irrespective of the technology used, the non-greenhouse gas emissions of the fuel cell system are much lower than both engine based technologies.
The figures of Table 12 are similar for CHP systems in residential applications, where primary energy savings and CO2 emission reductions are above 20% when using 1 kWe fuel cell CHP systems instead of using electricity from the grid and generating heat with boilers.122
7. Conclusions
Fuel cells are in development for a variety of applications. Their use in transport and for combined heat and power generation offers a great opportunity to save an appreciable amount of energy, while at the same time reducing emissions of non-greenhouse gases. As the technology of fuel cells becomes more mature, fuel cell vehicles as well as stationary power systems are available for field tests. Commercial introduction can only take place when reliability is proven and cost is reduced. A considerable improvement on materials, component and system level is to be expected before and during the widespread application of fuel cells.References
- At last, the fuel cell, The Economist, October 23rd, 1997 Search PubMed.
- T. Klaiber, J. Power Sources, 1996, 61, 61 CrossRef CAS.
- Estimated Economic Impacts and Market Potential Associated with the Development and Production of Fuel Cells in British Columbia, KPMG report for Ministry of Environment, Lands and Parks, Victoria, BC, Canada, 1996 Search PubMed.
- A. Casanova, J. Power Sources, 1998, 71, 65 CrossRef CAS.
- Y. Watanabe, M. Matsumoto and K. Takasu, J. Power Sources, 1996, 61, 53 CrossRef CAS.
- X. Ren, P. Zelenay, S. Thomas, J. Davey and S. Gottesfeld, J. Power Sources, 2000, 86, 111 CrossRef CAS.
- H. Dohle, H. Schmitz, T. Bewer, J. Mergel and D. Stolten, J. Power Sources, 2002, 106, 313 CrossRef CAS.
- R. D. Breault, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 59 Search PubMed.
- F. Barbir and T. Gomez, Int. J. Hydrogen Energy, 1996, 21, 891 CrossRef CAS.
- U. Bossel, Well-to-Wheel Studies, Heating Values, and the Energy Conservation Principle, European Fuel Cell Forum, 29th October 2003, available at http://www.efcf.com Search PubMed.
- M. A. J. Cropper, S. Geiger and D. M. Jollie, J. Power Sources, 2004, 131, 57 CrossRef CAS.
- D. E. Curton, R. D. Lousenberg, T. J. Henry, P. C. Tangeman and M. E. Tisack, J. Power Sources, 2004, 131, 41 CrossRef.
- A. V. Anantaraman and C. L. Gardner, J. Electroanal. Chem., 1996, 414, 115 CrossRef CAS.
- R. K. A. M. Mallant, J. Power Sources, 2003, 118, 424 CrossRef CAS.
- J. A. Kolde, B. Bahar and M. S. Wilson, in Proceedings of the 1st International Symposium on Proton Conducting Membrane Fuel Cells (1995), The Electrochemical Society, Pennington, NJ, USA, 1995, vol. 95-23, p. 193 Search PubMed.
- W. Liu, K. Ruth and G. Rusch, J. New Mater. Electrochem. Syst., 2001, 4, 227 Search PubMed.
- D. J. Jones, M. El Haddad, B. Mula and J. Rozière, Environ. Res. Forum, 1996, 1–2, 115–126 Search PubMed.
- A. E. Steck and C. Stone, Development of the BAM Membrane for Fuel Cell Applications, in New Materials for Fuel Cell and Modern Battery Systems II, ed. O. Savogado and P. R. Roberge, Ecole Polytechnique de Montreal, Montreal, Quebec, 1997, p. 792 Search PubMed.
- F. Helmer-Metzmann, F. Osan, A. Schneller, H. Ritter, K. Ledjeff, R. Nolte and R. Thorwirth, Polymer Electrolyte Membrane, and Process for the Production Thereof, US Pat., 5 438 082, 1995 (Hoechst) Search PubMed.
- J. Wei, C. Stone and A. E. Steck, Trifluorostyrene and Substituted Trifluorostyrene Copolymeric Compositions and Ion-Exchange Membranes Formed Therefrom, US Pat, 5 422 411 Search PubMed.
- F. N. Büchi, B. Gupta, O. Haas and G. G. Scherer, Electrochim. Acta, 1995, 40, 345–353 CrossRef CAS.
- S. Faure, N. Cornet, G. Gebel, R. Mercier, M. Pineri and B. Sillion, in New Materials for Fuel Cell and Modern Battery Systems II, ed. O. Savogado and P. R. Roberge, Ecole Polytechnique de Montreal, Montreal, Quebec, 1997, p. 828 Search PubMed.
- F. A. de Bruijn, D. C. Papageorgopoulos, E. F. Sitters and G. J. M. Janssen, J. Power Sources, 2002, 110, 117 CrossRef CAS.
- R. Savinell, E. Yeager, D. Tryk, U. Landau, J. Wainright, D. Weng, K. Lux, M. Litt and C. Rogers, J. Electrochem. Soc., 1994, 141, L46.
- Q. Li, R. He, J. O. Jensen and N. J. Bjerrum, J. Electrochem. Soc., 2003, 150, A1599 CrossRef CAS.
- O. Savogado, J. Power Sources, 2004, 127, 135 CrossRef.
- Q. Li, J. O. Jensen, R. He and N. J. Bjerrum, in Proceedings of the 1st European Hydrogen Energy Conference, Grenoble September 2–5, 2003, Association Française de l’Hydrogene, Paris, 2003 Search PubMed.
- K. V. Lovell and N. S. Page, Membrane Electrolyte Technology for Solid Polymer Fuel Cells, report ETSU F/02/00110/REP, Cranfield University, Bedford, UK, 1997 Search PubMed.
- T. R. Ralph, Platinum Met. Rev., 1997, 41, 102 CAS.
- C. G. M. Quah, Hydrogen Fuel Cell Lett., 1998, 8(4), 1–3 Search PubMed.
- D. M. Bernardi and M. W. Verbrugge, J. Electrochem. Soc., 1992, 139, 2477 CAS.
- H. A. Gasteiger, J. E. Panels and S. G. Yan, J. Power Sources, 2004, 127, 162 CrossRef CAS.
- K. Sasaki, J. X. Wang, M. Balasubramanian, J. McBreen, F. Uribe and R. R. Adzic, Electrochim. Acta, 2004, 49, 3873 CrossRef CAS.
- T. R. Ralph and M. P. Hogarth, Platinum Met. Rev., 2002, 46, 117 CAS.
- R. J. Bellows, E. P. Marucchi-Soos and D. T. Buckley, Ind. Eng. Chem. Res., 1996, 35, 1235 CrossRef CAS.
- S. D. Knights, K. M. Colbow, J. St-Pierre and D. P. Wilkinson, J. Power Sources, 2004, 127, 127 CrossRef CAS.
- D. P. Wilkinson and D. Thompsett, in New Materials for Fuel Cell and Modern Battery Systems II, ed. O. Savogado and P. R. Roberge, Ecole Polytechnique de Montreal, Montreal, Quebec, 1997, p. 266 Search PubMed.
- T. R. Ralph and M. P. Hogarth, Platinum Met. Rev., 2002, 46, 3 CAS.
- M. S. Wilson, J. A. Valerio and S. Gottesfeld, Electrochim. Acta, 1995, 40, 355 CrossRef CAS.
- A. Heinzel, F. Mahlendorf, O. Niemzig and C. Kreuz, J. Power Sources, 2004, 131, 35 CrossRef CAS.
- R. C. Makkus, A. H. H. Janssen, F. A. de Bruijn and R. K. A. M. Mallant, J. Power Sources, 2000, 86, 274 CrossRef CAS.
- J. Scholta, B. Rohland and J. Garche, in New Materials for Fuel Cell and Modern Battery Systems II, ed. O. Savogado and P. R. Roberge, Ecole Polytechnique de Montreal, Montreal, Quebec, 1997, p. 330 Search PubMed.
- C. Zawodzinski, M. S. Wilson and S. Gottesfeld, in 1996 Fuel Cell Seminar Orlando, FL, Courtesy Associates, Washington, DC, 1996, p. 659 Search PubMed.
- J. Newton, S. E. Foster, D. Hodgson and A. Marrett, Routes to Commercially Viable PEM Fuel Cell Stack, ETSU F/02/00155/REP, available at http://www.dti.gov.uk/energy/renewables/publications Search PubMed.
- N. Cunningham, D. Guay, J. P. Dodelet, Y. Meng, A. R. Hlil and A. S. Hay, J. Electrochem. Soc., 2002, 149, A905 CrossRef CAS.
- E. Middelman, W. Kout, B. Vogelaar, J. Lenssen and E. de Waal, J. Power Sources, 2003, 118, 44 CrossRef CAS.
- E. J. Carlsson, in Hydrogen, Fuel Cells and Infrastructure Technologies, 2003 Annual Progress report, US Department of Energy, Energy Efficiency and Renewable Energy, p IV-13 Search PubMed.
- G. Hards, J. Buchanan, T. Ralph, C. de Rouffignac, J. Rowe and D. Thompsett, in 2002 Fuel Cell Seminar Abstracts, Courtesy Associates, Washington, DC, 2002, p. 854 Search PubMed.
- D. Wheeler, T. Clark and S. Motupally, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 774 Search PubMed.
- S. Cleghorn, J. Kolde, R. Reid and O. Teller, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 832 Search PubMed.
- Product sheet Ballard Mark 902, available at http://www.ballard.com, July 2004.
- Product sheet General Motors, GM Fuel Cell Technology for Sustainable Mobility, Adam Opel AG, Rüsselsheim, 2004 Search PubMed.
- Product sheet Teledyne Energy Systems, available at http://www.teledynees.com, July 2004.
- A. Folkesson, C. Andersson, P. Alvfors, M. Alaküla and L. Overgaard, J. Power Sources, 2003, 118, 349 CrossRef CAS.
- D. P. Wilkinson, in The Electrochemical Society Interface, Spring 2001, The Electrochemical Society, Pennington, NJ, 2001, p. 22 Search PubMed.
- M. Fowler, J. C. Amphlett, R. F. Mann, B. A. Peppley and P. R. Roberge, J. New Mater. Electrochem. Syst., 2002, 5, 255 Search PubMed.
- C. Handley, N. P. Brandon and R. van der Vorst, J. Power Sources, 2002, 106, 344 CrossRef CAS.
- F. Uribe, T. Zawodzinski and S. Gottesfeld, ECS Proc., 1998, 98-27, 229 Search PubMed.
- J. B. J. Veldhuis, F. A. de Bruijn and R. K. A. M. Mallant, in 1998 Fuel Cell Seminar Abstracts, Palm Springs, Courtesy Associates, Washington, DC, 1998, p. 598 Search PubMed.
- L. Pino, V. Recupero, M. Lagana and M. Minutoli, in 1998 Fuel Cell Seminar Abstracts, Courtesy Associates, Washington, DC, 1998, p. 671 Search PubMed.
- J. St.-Pierre and N. Jia, J. New Mater. Electrochem. Syst., 2002, 5, 263 Search PubMed.
- E. Cho, J.-J. Ko, H. Y. Ha, S.-A. Hong, K.-Y. Lee, T.-W. Lim and I.-H. Oh, J. Electrochem. Soc., 2004, 151, A661 CrossRef CAS.
- M. Wakizoe, H. Murata and H. Takei, in 1998 Fuel Cell Seminar Abstracts, Palm Springs, CA, Courtesy Associates, Washington, DC, 1998, abstract 1140 Search PubMed.
- P. J. de Wild, R. G. Nyqvist and F. A. de Bruijn, in 2002 Fuel Cell Seminar Abstracts, Courtesy Associates, Washington, DC, 2002, p. 227 Search PubMed.
- S. Ibe, K. Hirai, N. Shinke, O. Yamazaki, S. Higashiguchi, K. Yasuhara, M. Hamabashiri and T. Tabata, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 941 Search PubMed.
- T. Kawada and J. Mizusaki, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 70 Search PubMed.
- A. Weber and E. Ivers-Tiffée, J. Power Sources, 2004, 127, 273 CrossRef CAS.
- G. J. Saunders, J. Preece and K. Kendall, J. Power Sources, 2004, 131, 23 CrossRef CAS.
- G. Rietveld, P. Nammensma, J. P. Ouweltjes, G. van Druten and R. Huiberts, in 2002 Fuel Cell Seminar, Portland, Courtesy Associates, Washington, DC, 2002, p. 886 Search PubMed.
- O. A. Marina, J. S. Hardy, G. W. Coffrey, S. P. Simner and K. D. Meinhardt, in 2002 Fuel Cell Seminar, Portland, Courtesy Associates, Washington, DC, 2002, p. 295 Search PubMed.
- O. Yamamoto, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 71 Search PubMed.
- H. Tu and U. Stimmung, J. Power Sources, 2004, 127, 284 CrossRef CAS.
- D. Stöver, H. P. Buchkremer and J. P. P. Huijsmans, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 72 Search PubMed.
- K. Hilpert, W. J. Quadakkers and L. Singheiser, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 74 Search PubMed.
- Z. Yang, S. Weil, D. M. Paxton and J. W. Stevenson, J. Electrochem. Soc., 2003, 150, A1188 CrossRef CAS.
- K. Fujita, K. Ogasawara, Y. Matsuzaki and T. Sakurai, J. Power Sources, 2004, 131, 261 CrossRef CAS.
- N. Dekker, G. Rietveld, J. Laatsch and F. Tietz, in 6th European Solid Oxide Fuel Cell Forum, Lucerne, 28 June–2 July 2004, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2004, p. 319.
- D. Ghosh, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p.135 Search PubMed.
- J. Zizelman, C. DeMinco, S. Mukerjee, J. Tachtler, J. Kammerer and P. Lamp, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p.306 Search PubMed.
- R. Steinberger-Wilckens, I. C. Vinke, L. Blum, J. Remmel, F. Tietz and W. J. Quadakkers, in 6th European Solid Oxide Fuel Cell Forum, Lucerne, 28 June–2 July 2004, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2004, p. 11.
- S. Shaffer, Development Update on Delphi's Solid Oxide Fuel Cell System, 2004 SECA Review Meeting, Boston, available at: http://www.netl.doe.gov Search PubMed.
- J. Dinsdale, K. Foger, J. Love, R. Ratnaraj and A. Washusen, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 884 Search PubMed.
- R. A. George, SECA Project at Siemens Westinghouse, 3rd Annual Solid State Energy Conversion Alliance Workshop, March 21–22, 2002 Search PubMed.
- N. P. Brandon, S. Skinner and B. C. H. Steele, Annu. Rev. Mater. Res., 2003, 33, 183 Search PubMed.
- S. C. Singhal, Solid State Ionics, 2002, 152–153, 405 CrossRef CAS.
- M. C. Willliams, J. P. Strakey and S. C. Singhal, J. Power Sources, 2004, 131, 79 CrossRef.
- N. Minh, Solid Oxide Fuel Cell Technology for Hybrid Power Generation, 2nd DoE/UN International Conference and Workshop on Hybrid Power Systems, April 2002, available at: http://www.netl.doe.gov Search PubMed.
- G. Israelson, J. Mater. Eng. Perform., 2004, 13, 282 Search PubMed.
- B. Borglum and E. Neary, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 786 Search PubMed.
- J. Hansen, J. Pålsson, J. Nielsen, E. Fontell, T. Kivisaari, P. Jumppanen and P. Hendriksen, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 790, and presentation available at http://www.fuelcellseminar.com Search PubMed.
- K. Konishi, J. Iritani, N. Komiyama, T. Kabata, N. Hisatome, K. Nagata and K. Ikeda, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 95 Search PubMed.
- R. Wustler and J. Schindler, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 3, ch. 5 Search PubMed.
- Hydrogen, Fuel Cells and Infrastructure Technologies, 2003 Annual Program Report, US Department of Energy, Energy Efficiency and Renewable Energy, Washington, DC Search PubMed.
- A. Bouza, C. J. Read, S. Satyapal and J. Milliken, 2004 Annual DoE Hydrogen Program Review–Hydrogen Storage, available at: http://www.eere.energy.gov..
- M. M. Herrmann and J. Meusinger, 1st European Hydrogen Energy Conference, Grenoble 2–5 September 2003, paper CO2-175.
- J. Wolf, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 3, ch. 7 Search PubMed.
- R. Harris, D. Book, P. Anderson and P. Edwards, Fuel Cell Rev., 2004, 1, 17 Search PubMed.
- A. Züttel, in Proceedings of the International German Hydrogen Congress, 11–12 February 2004, Essen, ee energy engineers, Essen, Germany, 2004.
- European Fuel Cell and Hydrogen Projects 1999–2002, Directorate-General for Research, EUR 20718, Brussels 2003 Search PubMed.
- D. zur Megede, J. Power Sources, 2002, 106, 35 CrossRef CAS.
- B. Eliasson and U. Bossel, in Proceedings of The Fuel Cell World, Lucerne, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 367 Search PubMed.
- F. Panik and O. Vollrath, in Proceedings of The Fuel Cell World, Lucerne, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 196 Search PubMed.
- M. Krumpelt, J. D. Carter, R. Wilkenhoener, S. H. D. Lee, J.-M. Bae and S. Ahmed, in 2000 Fuel Cell Seminar, Portland, Courtesy Associates, Washington, DC, 2000, p. 542 Search PubMed.
- P. S. Chintawar, B. Bowers, C. O'Brien, Z.-Y. Xue, J. Cross and W. Mitchell, in Hydrogen, Fuel Cells and Infrastructure Technologies, 2003 Annual Progress Report, US Department of Energy, Energy Efficiency and Renewable Energy, p IV-157 Search PubMed.
- DoE Decision Team Committee Report, On-Board Fuel Processing Go/No Decision, August 2004, available at: http://www.eere.energy.gov/hydrogenandfuelcells/.
- M. Echigo, N. Shinke, S. Takami and T. Tabata, J. Power Sources, 2004, 132, 29 CrossRef CAS.
- J. Komiya, N. Fujiwara, H. Fujiki, T. Miura and I. Yasuda, in 2002 Fuel Cell Seminar, Palm Springs, Courtesy Associates, Washington, DC, 2002, p. 942 Search PubMed.
- W. L. Mitchell, in 2002 Fuel Cell Seminar, Palm Springs, Courtesy Associates, Washington, DC, 2002, p. 952 Search PubMed.
- A. Fuju, K. Shindo, O. Tajima and H. Izaki, in 2002 Fuel Cell Seminar, Palm Springs, Courtesy Associates, Washington, DC, 2002, p. 616 Search PubMed.
- M. J. Binder, W. R. Taylor and F. H. Holcomb, 2001 International Gas Research Conference Amsterdam, the Netherlands.
- T. Fukunaga, H. Katsuno, H. Matsumoto, O. Takahashi and Y. Akai, Catal. Today, 2003, 84, 197 CrossRef CAS.
- B. Davenport, Plug Power Demonstration Project, United States Military Acadamy West Point, NY, DACA 42-03-C-0005 Search PubMed.
- Site performance of residental fleet on http://www.dodfuelcell.com/res.
- T. Mizukami, T. Okusawa, K. Takahashi, N. Imada and Y. Enokizu, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 1 Search PubMed.
- H. Horinouchi, N. Osaka, J. Miyake, M. Kawamura, T. Miura and K. Nishizaki, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 17 Search PubMed.
- I. Nakagawa, T. Kiyota, Y. Chida and T. Koike, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 81 Search PubMed.
- Information on Millenium project available at http://www.pefc.net.
- Z. Barisic, in Proceedings of The Fuel Cell World, Lucerne, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 105 Search PubMed.
- H. Raak, R. Diethelm and S. Riggenbach, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 81 Search PubMed.
- R. George and A. Casanova, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 895 Search PubMed.
- A. Schuler, J. Schild, E. Batawi, A. Rüegge, M. Tamas, T. Doerk, H. Raak and B. Doggwiler, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 128 Search PubMed.
- P. F. van den Oosterkamp and P. C. van der Laag, Proceedings AIChE Spring meeting, New Orleans 2003, paper 98G.
- B. Borglum and E. Neary, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 786 Search PubMed.
- K. Konishi, J. Iritani, N. Komiyama, T. Kabata, N. Hisatome, K. Nagata and K. Ikeda, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p.95 Search PubMed.
- E. Fontell, T. Kivisaari, N. Christansen, J.-B. Hansen and J. Pålson, J. Power Sources, 2004, 131, 49 CrossRef CAS.
- J. Zizelman, S. Shaffer and S. Mukerjee, in 2003 Fuel Cell Seminar, Miami, Courtesy Associates, Washington, DC, 2003, p. 888 Search PubMed.
- http://www.fuelcells.org.
- http://www.fuelcellpartnership.org/ .
- F. Büchi, A. Tsukada, P. Rodatz, O. Garcia, M. Ruge, R. Kötz, M. Bärtschi and P. Dietrich, in Proceedings of The Fuel Cell World, Lucerne, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 218 Search PubMed.
- A. Martin, in Proceedings of The Fuel Cell World, Lucerne, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 232 Search PubMed.
- R. F. Service, The Hydrogen Backlash, Science, 2004, 305, 958 Search PubMed.
- G. F. McLean, T. Niet, S. Prince-Richard and N. Djilali, Int. J. Hydrogen Energy, 2002, 27, 507 CrossRef CAS.
- M. Cifrain and K. V. Kordesch, J. Power Sources, 2004, 127, 234 CrossRef CAS.
- M. Schulze and E. Gülzow, J. Power Sources, 2004, 127, 252 CrossRef CAS.
- E. Gülzow and M. Schulze, J. Power Sources, 2004, 127, 243 CrossRef CAS.
- K. Kordesch, V. Hacker, J. Gsellmann, M. Cifrain, G. Faleschini, P. Enzinger, R. Fankhauser, M. Ortner, M. Muhr and R. R. Aronson, J. Power Sources, 2000, 86, 162 CrossRef CAS.
- Y. Mugikura, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 66 Search PubMed.
- G. Huppmann, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 171 Search PubMed.
- T. Bardewyck, in Proceedings of The Fuel Cell World, Lucerne, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 102 Search PubMed.
- R.-H. Song, S. Dheenadayalan and D.-R. Shin, J. Power Sources, 2002, 106, 167 CAS.
- D. A. Landsman and F. J. Luczak, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, UK, 2003, vol. 4, ch. 60 Search PubMed.
- D. Rastler, in Proceedings of The Fuel Cell World, 1–5 July 2002, ed. M. Nurdin, European Fuel Cell Forum, Oberrohrdorf, Switzerland, 2002, p. 383 Search PubMed.
- R. D. Breault, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm and H. A. Gasteiger, Wiley, Chichester, 2003, vol. 4, ch. 59 Search PubMed.
- Site performance of PAFC fleet on http://www.dodfuelcell.com/pafc.
- J. C. Yang, Y. S. Park, S. H. Seo, H. J. Lee and J. S. Noh, J. Power Sources, 2002, 106, 68 CrossRef CAS.
- World Energy Investment Outlook 2003, International Energy Agency, IEA Publications, Paris.
- K. Kasahara, M. Morioka, H. Yoshida and H. Shingai, J. Power Sources, 2000, 86, 298 CrossRef CAS.
- European Directive 1999/96/EC, from http://www.dieselnet.com.
- http://www.epa.gov.
- GM Well to Wheel Analysis of Energy Use and Greenhouse Gas Emissions of Advanced Fuel/Vehicle Systems—a European Study, L-B Systemtechnik GmbH, Ottobrun, 27 September 2002, available at http://www.lbst.de/gm-wtw. Search PubMed.
- M. A. Weiss, J. B. Heywood, A. Schafer and V. K. Natarajan, MIT report LFEE 2003-001 RP, available at: http://lfee.mit.edu/publications/PDF/LFEE_2003-001_RP.pdf.
- Emission data available at http://www.emis.vito.be.
- http://www.dti.gov.uk.
- Prius Green Report, Toyota Motor Corporation, June 2003, available at: http://www.toyota.co.jp/en/k_forum/tenji/pdf/pgr_e.pdf.
- D. Basteels and R. A. Searles, Platinum Met. Rev., 2002, 46, 27.
- G. Erdmann, Int. J. Hydrogen Energy, 2003, 28, 685 CrossRef CAS.
- D. Hart and A. Bauen, Further Assessment of the Environmental Characteristics of Fuel Cells and Competing Technologies, ETSU F/02/00153/REP, Imperial College, London, 1998 Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |

