Microwave-promoted synthesis of β-hydroxy sulfides and β-hydroxy sulfoxides in water
Vincenza
Pironti
* and
Stefano
Colonna
Istituto di Chimica Organica, “A. Marchesini”, Facoltà di Farmacia, Università degli Studi di Milano, via Venezian, 21, 20133, Milano, Italy. E-mail: Vincenza.Pironti@unimi.it; Fax: +390250314476; Tel: +390250314478
First published on 3rd December 2004
Abstract
A simple, efficient and environmentally friendly one-pot synthesis of β-hydroxy sulfoxides in water under microwave irradiation is reported.
Introduction
β-Hydroxy sulfides and β-hydroxy sulfoxides are important intermediates in organic synthesis.1The former are versatile building blocks for synthesizing cyclic sulfides,2 allylic alcohols,1 benzoxathiepines,3 benzothiazepines4 and thioketones.5 On the other hand the readily accessible β-hydroxy sulfoxides have an extended use in asymmetric synthesis.6 They find also a useful application in the preparation of naturally occurring compounds such as leukotrienes.7
The most important synthetic route to β-hydroxy sulfide is the ring opening of epoxides with thiolates.7 It can be promoted by acids, Lewis catalyst or metal salts.8 Thiolysis in water was investigated for the first time by Fringuelli and coworkers,9 where the formation of β-hydroxy sulfides was promoted by Lewis acids such as InCl39 and ZnCl2.10
β-Hydroxy sulfoxides are commonly prepared by oxidation of β-hydroxy sulfides with conventional oxidizing agents.
We herein report the optimization of a one-pot protocol for the preparation of β-hydroxy sulfoxides in water, promoted by microwave irradiation. The opening of the epoxide and the subsequent sulfoxidation were accelerated by microwave and the desired product was obtained in only 15 minutes with very good yield. (Scheme 1) (see Table 1). Microwave activation as a non-conventional energy source has become an important method that can be used to carry out a wide range of reactions with short reaction times and in high yield and regioselectivity.11 Indeed microwave dielectric heating in a pressurized system rapidly increases temperature far above the boiling point of the solvent and leads to a uniform energy transfer to the reactants of the chemical reaction.
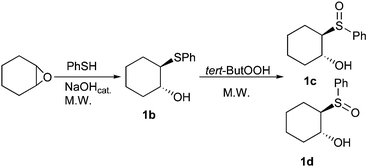 | ||
| Scheme 1 Example of one-pot synthesis. | ||
| Entry | Epoxide | T/°C | Time/min | Power/Watt | Yield (%) | a/ba |
|---|---|---|---|---|---|---|
| a Refers to the ratio of a-carbon and b-carbon, determined by 1H-NMR and HPLC analyses. | ||||||
| 1 |
 1a
1a
|
25 | 240 | — | 80 | |
| 2 | 1a | 150 | 5 | 400 | 97 | |
| 3 | 1a | 110 | 5 | — | 30 | |
| 4 |
 2a
2a
|
25 | 300 | — | 72 | |
| 5 | 2a | 150 | 5 | 400 | 95 | |
| 6 |
 3a
3a
|
25 | 1440 | — | 43 | |
| 7 | 3a | 150 | 10 | 400 | 91 | |
| 8 |
 4a
4a
|
25 | 300 | — | 77 | 5/95 |
| 9 | 4a | 150 | 5 | 400 | 85 | 3/97 |
| 10 |
 5a
5a
|
25 | 270 | — | 72 | 80/20 |
| 11 | 5a | 150 | 5 | 400 | 88 | 65/35 |
| 12 |
 6a
6a
|
25 | 180 | — | 85 | 5/95 |
| 13 | 6a | 150 | 5 | 400 | 98 | 3/97 |
Water is cheap, readily available and nontoxic resulting in an economic process and has clear advantages as an environmentally friendly solvent alternative in organic synthesis. The heating effect utilized in microwave-assisted organic transformations is due to the dielectric constant of the solvent. For this reason, water is therefore a very useful solvent for microwave-assisted organic synthesis.11
Results and discussion
The reaction was performed with a laboratory microwave oven in a sealed tube controlled at 150 °C with an optical fiber in the presence of a catalytic amount of sodium hydroxide12 (1.5%). The epoxide and the sulfide were used in a stoichiometric ratio. The β-hydroxy sulfide was obtained in quantitative yields (for reaction conditions see Table 1). In the one-pot procedure tert-butyl hydroperoxide (2.0 equiv.) was added to the same reaction media at 100 °C leading to β-hydroxy sulfoxides 1c and 1d in very good yields. No appreciable amounts of the corresponding sulfone were found.This reaction was optimized using epoxide 1a as starting material and thiophenol. Different parameters such as temperature, equivalent of oxidant and reaction time were studied (see Table 2). The β-hydroxy sulfoxides were isolated in 89% yield as a 25 ∶ 75 mixture of diastereoisomers 1c and 1d of known configuration1,9 (see Scheme 1).
| Entry | tert-ButOOH/mol equiv.−1 | T/°C | Time/min | Power/Watt | 1c ∶ 1d | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 3.0 | 25 | 720 | — | 35 ∶ 65 | 60 |
| 2 | 3.0 | 70 | 330 | — | 30 ∶ 70 | 65 |
| 3 | 1.0 | 70 | 10 | 400 | 29 ∶ 71 | 34 |
| 4 | 1.0 | 70 | 15 | 400 | 31 ∶ 69 | 43 |
| 6 | 2.0 | 100 | 15 | 400 | 25 ∶ 75 | 89 |
In order to extend our protocol we have also investigated the thiolysis of a variety of epoxides (1a–6a) with thiophenol.
When the thiolysis was performed with microwave irradiation the reaction was very rapid and the products isolated from the reaction media were pure and did not need further purification.
At room temperature and in the absence of microwave irradiation the reaction times were in the range of 3–24 hours and the yields were lower (Table 1).
The rate acceleration observed can be justified as a consequence of the higher temperature and of the microwave irradiation. The opening of 1a performed with conventional heating at 110 °C provided the expected product 1b with only 30% yield (see Table 1 entry 3).
In all cases the ring opening was completely anti-stereoselective and the only products obtained were the trans-β-hydroxy sulfides.13 The reaction proceeded via an SN2 mechanism with attack of the thiol at the less substituted b-carbon, except for compound 5a where the attack was driven predominantly at the benzylic a-carbon by an electronic effect.
Conclusion
In conclusion we have demonstrated that the thiolysis of several epoxides in water, under controlled microwave irradiation, can be performed in a short time and with quantitative yield without the use of any metal catalyst. By coupling this procedure with the in situ oxidation of sulfide mediated by tert-butyl hydroperoxide, we have optimized a one-pot procedure for the preparation of β-hydroxy sulfoxides.Experimental
Typical procedure of opening of an epoxide 1a–6a in the presence of thiophenol
Thiophenol (1.5 mmol) and epoxide (1.5 mmol) were suspended in water (3 ml), then sodium hydroxide (0.022 mmol) was added . The reaction tube (25 ml) was sealed with a Teflon® cap and irradiated in the cavity of a multimode Milestone MicroSYNTH labstation for the appropriate time at 150 °C, using an irradiation power of 400 Watt. The tube was cooled to 50 °C by gas-jet cooling. The reaction mixture was extracted with diethyl ether (3 × 5 ml). The combined organic layers were dried on magnesium sulfate and removed under reduced pressure to give pure product.Optimized procedure for one-pot synthesis of β-hydroxy sulfoxide
Thiophenol (1.5 mmol.) and epoxycyclohexane (1.5 mmol) were suspended in water then sodium hydroxide was added (0.022 mmol). The reaction tube was sealed with a Teflon® cap and irradiated in the cavity of a microwave apparatus at 150 °C for 5 minutes. The reaction tube was cooled to room temperature by gas-jet cooling, tert-ButOOH (3.0 mmol) was added and then irradiated in the cavity of microwave apparatus at 100 °C using an irradiation power of 400 Watt. After the appropriate reaction time the tube was cooled to 50 °C by gas-jet cooling. The reaction mixture was extracted with diethyl ether (3 × 5 ml). The combined organic layers were dried on magnesium sulfate and the solvent was removed under reduced pressure to give the β-hydroxy sulfoxides as a mixture of diastereoisomers.Acknowledgements
This work was supported by a PRIN project of the Italian MIUR. We want to thank Dr. Giorgio Abbiati and Dr.ssa Alessandra Bellinazzi for their help with the microwave apparatus.References
- V. Kesavan, D. Bonnet-Delpon and J. P. Bégué, Tetrahedron Lett., 2000, 41, 2895 CrossRef and references therein.
- (a) E. Ozaki, H. Matsui, H. Yoshinaga and S. Kitagawa, Tetrahedron Lett., 2000, 41, 2621 CrossRef; (b) B. M. Adger, J. V. Barkley, S. Bergeron, M. W. Cappi, B. E. Flowerdew, M. P. Jackson, R. McCague, T. C. Nugent and S. M. Roberts, J. Chem. Soc., Perkin Trans. 1, 1997, 3501 RSC.
- (a) H. Sugihara, H. Mabuchi, M. Hirata, T. Iamamoto and Y. Kawamatsu, Chem. Pharm. Bull., 1987, 35, 1930 CAS; (b) C. G. Aifheli and P. T. Kaye, Synth. Commun., 1996, 26, 4459; (c) R. Krishnamutri, S. Nagy and T. F. Smolka, US Patent NO 5621153, 1997 Search PubMed.
- A. Scwartz, P. B. Madan, E. Mohacsi, J. P. O'Brien, E. Todaro and D. L. Coffen, J. Org. Chem., 1992, 57, 851 CrossRef CAS.
- J. P. Bégué, D. Bonnet-Delpon and A. Kornilov, Synthesis, 1996, 529 CAS.
- (a) M. C. Carreňo, Chem. Rev., 1995, 95, 1717 CrossRef CAS; (b) M. C. Carreňo, M. Ribagorda and G. H. Posner, Angew. Chem., Int. Ed., 2002, 41, 2753 CrossRef CAS and references therein.
- E. J. Corey, D. A. Clark, G. Goto, A. Marfat, C. Mioskowski, B. Samuelsson and S. Hammarstrom, J. Am. Chem. Soc., 1980, 102, 1436 CrossRef CAS.
- (a) A. E. Vougiokas and H. B. Kagan, Tetrahedron Lett., 1987, 28, 6065 CrossRef CAS; (b) J. Iqbal, A. Pandey, A. Shukla, R. R. Srivastava and S. Tripathi, Tetrahedron, 1990, 18, 6423 CrossRef CAS; (c) K. S. Ravikumar, F. Barbier, J. P. Bégué and D. Bonnet-Delpon, J. Fluorine Chem., 1999, 95, 123 CrossRef CAS.
- F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, Adv. Synth. Catal., 2002, 344, 379 CrossRef CAS.
- (a) D. Amantini, F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, Synlett, 2003, 15, 2292; (b) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, J. Org. Chem., 2003, 68, 8248 CrossRef CAS.
- Microwaves in Organic Synthesis, ed. A. Loupy, Wiley-VCH, Weinheim, 2002 Search PubMed.
- In the absence of a catalytic amount of sodium hydroxide, compound 1b was obtained in 13% yield.
- The trans configuration was deduced from the JH–H coupling constants: for more information see ref. 1 .
| This journal is © The Royal Society of Chemistry 2005 |
