Sea-urchin (Paracentrotus lividus) glutathione S-transferases and cholinesterase activities as biomarkers of environmental contamination
Isabel
Cunha
*a,
Luz Maria
García
b and
Lúcia
Guilhermino
ac
aCIIMAR–Centro Interdisciplinar de Investigação Marinha e Ambiental, Laboratório de Ecotoxicologia, Rua dos Bragas, 289, 4050-123, Porto, Portugal. E-mail: isabel.cunha@cimar.org.; Fax: (+351) 22 33 90 608; Tel: (+351) 22 34 01 800
bCIAD–Centro de Investigacion para la Alimentación y el Desarrollo, Ecotoxicology Laboratory, Sábalo Cerritos s/n Estero de Yugo, CP 82010, apartado postal 711, Mazatlán, Sinaloa, Mexico
cICBAS–Instituto de Ciências Biomédicas de Abel Salazar, Departamento de Estudos de Populações, Laboratório de Ecotoxicologia, Universidade do Porto, Lg. Prof. Abel Salazar, 2, 4099-003 Porto, Portugal
First published on 3rd March 2005
Abstract
Activities of glutathione S-transferases (GST) and cholinesterase (ChE) from Paracentrotus lividus were investigated as possible biomarkers of environmental contamination in the coastal zone. In the first phase of the study, the activity of both enzymes was determined in various tissues in order to select the most appropriate ones to be used in the following assays. In the second phase, the ChEs present in ambulacra were characterized using different substrates and selective inhibitors. In the next phase, laboratory bioassays were performed with dilutions of water-accommodated fraction of #4 fuel-oil (WAF) and benzo[a]pyrene (BaP) to determine the response of those enzymes to these pollutants and, finally, the activity of both enzymes was determined during a year in indigenous specimens from six sites on the Northwest coast of Portugal, with different pollution levels, to determine basal values and seasonal variations of ChE and GST activities. Among the several tissues tested, ambulacra and the anterior portion of the intestine were selected for ChE and GST assays, respectively. The determination of ChE in ambulacra tissue may be performed in a non-destructive way. Ambulacra ChE hydrolysed acetylthiocholine preferentially to propionylthiocholine and butyrylthiocholine and, inhibition by excess of substrate was observed. Enzymatic activity was almost fully inhibited by eserine sulfate (>98%) at concentrations equal or higher than 6.25 μM. Sensitivity to both BW284C51 (reaching 98% at 200 μM) and iso-OMPA (73% at 8 mM) was found. In laboratory bioassays, GSTs activity was inhibited by WAF and induced by BaP, whereas ChE activity was not affected by any of these environmental contaminants. Seasonal variations in enzymatic activities were found. For example, in a reference site, ChE values changed from 52.2 ± 9.3 U mg−1 protein in autumn to 71.8 ± 13.3 U mg−1 protein in spring, while GST activity changed from 129.9 ± 29.8 U mg−1 protein in winter to 279.0 ± 48.0 U mg−1 protein in autumn. Sea-urchins from reference sites presented significantly higher values of both ChE and GST than animals from contaminated sites in all seasons. In conclusion, the results of this study indicate that (i) ambulacra and the anterior portion of intestine are the most suitable tissues to measure ChE and GST activities, respectively; (ii) only one form of ChE seems to be present in ambulacra, showing properties of both typical acetylcholinesterase (AChE) and pseudocholinesterase (PChE); (iii) P. lividus GST is sensitive to both WAF and BaP even after acute exposures while ChE is not, and (iv) in spite of the significant seasonal variations observed in both enzymes in the field, P. lividus ChE and GST were capable of discriminate sites with different contamination levels and, thus, they are suitable for use as biomarkers in biomonitoring studies in the coastal zone.
Introduction
Biomarkers may be used as early warning signals anticipating possible major disturbances at higher levels of organization and thus they may be of great value in risk assessment studies. In recent decades, several enzymes have been used as environmental biomarkers, including cholinesterases (ChE) and glutathione S-transferases (GST).ChEs are a group of esterases, which are present in all animal Phyla. ChEs of several species have been used as environmental biomarkers, including those of marine invertebrates living in the coastal zone, such as echinoderms.1 Similarly to the enzymes of other animals, ChE activity of marine invertebrates has also been found to be strongly inhibited by organophosphate and carbamate compounds.2–5 More recently, several environmental contaminants such as heavy metals5,6 and petroleum-derived products7 have also been reported to inhibit ChE of these organisms. In recent years, different ChE forms have been found in the same tissue of marine invertebrates8,9 Since different ChE forms may have different sensitivities to anticholinesterase agents present in the environment and, as this may be a possible source of error, it is important to characterise the ChE forms present in species and tissue selected for biomonitoring studies.
GSTs are a well-known phase II detoxification iso-enzymes family, catalysing the conjugation of glutathione with both xenobiotics and endogenous substances. GSTs are also important in preventing lipid peroxidation. These enzymes can be induced by certain xenobiotics such as polycyclic aromatic hydrocarbons (PAH), polycyclic biphenyls (PCBs) and phenobarbital compounds.10–12 In invertebrates, whole organism and particular organs and cells, GST activity has been shown to increase as a function of the concentration of some xenobiotics in seawater.13,14 Therefore, GST activity of marine invertebrates, specially of molluscs, has been used as an environmental biomarker (e.g. for PAH15,16 and metal17 contamination). However, the induction of GST in invertebrates is often difficult to measure and frequently shows patterns of difficult interpretation.
Biological responses of the organisms, including enzymatic activities, may be affected by temperature, salinity, hypoxia, food availability, reproductive cycle,18,19 as well as seasonal differences in bioavailability and bioaccumulation of contaminants.20 Therefore, before using enzymes of a different species as environmental biomarkers, the range of “normal activity” in the tissue to be used should be determined in organisms of a reference population and seasonal variations should be studied, in order to distinguish natural fluctuations from responses to pollutant exposure.
Paracentrotus lividus are predominantly herbivorous21 having important effects on the structure and dynamics of species assemblages in coastal habitats, including seagrass and Posidonia oceanica meadows, kelp beds, other assemblages of macroalgae and littoral rock pools.22–25P. lividus is the largest and most abundant echinoid along the Portuguese coast and is also well-distributed and abundant in other ecosystems all over the Mediterranean and northeast Atlantic coasts,26 both in “clean” and polluted areas. Therefore, this species seems to have potential to be used as a bioindicator in biomonitoring studies.
The central objective of the present study was to investigate the potential of the activity of GST and ChE of P. lividus to be used as biomarkers of environmental contamination in coastal areas. To attain this main goal, the following phases of study were considered: (i) determination of the activity of GSTs and ChE in several tissues of the sea-urchin to choose the most suitable tissues to use in further experiments, (ii) biochemical characterization of the ChE present in ambulacral podia homogenates to reduce errors due to the potential presence of distinct enzymatic forms with different sensitivity to anticholinesterase pollutants, (iii) laboratory bioassays with dilutions of the water-accommodated fraction (WAF) of a fuel-oil (#4) and benzo[a]pyrene (BaP) to standardise methodologies and to investigate responsiveness of GST and ChE to these products, (iv) determination of GST and ChE activities during a year in indigenous specimens from six sites on the North Atlantic coast of Portugal to determine ranges of activity, natural fluctuations of enzymatic levels and to evaluate the capability of these enzymes to discriminate sites with different pollution levels.
Materials and methods
Enzymatic activity in tissues
P. lividus specimens collected from a reference sampling station (S. Bartolomeu do Mar–SB) were used to determine ChE and GSTs basal activities in different tissues. Specimens of 35–40 mm-theca perimeter were collected during low tide from tide-pools. Upon arrival at the laboratory, animals were sacrificed, tissues collected and stored at −80 °C until assayed. Coelomatic fluid, coelomatic membrane, radial canal, Aristotle’s lantern muscles, ambulacral podia and intestine were tested for ChE and GSTs activities. In the case of GSTs, various sections of the intestine were separately tested: proximal, medium and distal, each part corresponding to one third of the total intestine length. In subsequent experiments, only ambulacra from the basal region (5–10 individual−1) and proximal intestine were used for ChE and GSTs, respectively.Enzymatic assays
Tissues for ChE and GSTs assays were homogenized in ice-cold phosphate buffer (0.1 M) at pH 7.2 and 6.5, respectively, using a politron (Ystral, D-79282). Homogenates were centrifuged at 3300 g for 10 min for ChE and at 9000 g for 30 min for GSTs. Supernatants were stored at −80 °C until assay. All samples were assayed within one week of collection. Enzymatic assays were performed at 25 °C.ChE activity was assayed at 414 nm using acetylthiocholine as substrate (except for characterization, when different substrates were used), according to Ellman’s method27 adapted to microplate,28 using a Labsystems Multiscan EX plate reader. A volume of 0.05 mL of supernatant to 0.25 mL of reaction solution was used. Reaction solution was 6.41 × 10−2 mM on 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and 2.5 mM on acetylthiocholine, in phosphate buffer.
GSTs activity was determined at 314 nm 29 using 1-chloro-2,4-dinitrobenzeno (CDNB) as substrate. A volume of 0.1 mL of supernatant to 0.2 mL of reaction solution was used. Reaction solution was 10 mM on CDNB and 60 mM in GSH, in phosphate buffer.
Enzymatic activities were expressed as units of activity (U) per mg of protein. Each unit of activity corresponded to 1 nmol of substrate hydrolysed per minute.
Characterization of cholinesterases
ChEs present in ambulacral podia were characterized by determining their substrate preferences and response to selective inhibitors. Substrates tested were acetylthiocholine (AcSCh), which is preferably hydrolysed by acetylcholinesterase, butyrylthiocholine (BuSCh) and propionylthiocholine (PrSCh), which are both preferentially hydrolysed by pseudocholinesterases. All of them were tested in a concentration range from 0.005 to 20.48 mM. Kinetic parameters for ChE substrates, Km and Vm, were obtained through hyperbolic regression analysis, where a non-linear regression fits the data directly to the best hyperbola. This approach seems to be preferable to methods which fit to linear transformations of the data because these can result in artificial weighting of data leading to erroneous estimates of Vm and Km.30 In the case of AcSCh, only values for concentrations smaller than 2.6 mM were considered for kinetic parameter determination, because inhibition of activity was observed at high substrate concentrations, deviating from the hyperbolic model.Inhibition of ChEs activity was assayed after in vitro incubation of homogenates for 30 min, in the presence of each inhibitor: eserine sulfate, an inhibitor of ChEs but not of other esterases; 1,5-bis(4-allydimethyl-ammoniumphenyl)pentan-3-one dibromide (BW284C51), considered as a selective inhibitor of acetylcholinesterase (AChE) and tetraisopropyl pyrophosphoramide (iso-OMPA), a selective inhibitor of pseudocholinesterases (PChE). ChEs activity was assayed immediately after incubation using AcSCh as substrate. Assays were performed at 25 °C, in triplicate. Each replicate was a pool of ambulacra collected from five animals.
Laboratory bioassays
Specimens of 35–40 mm-theca perimeter were collected from tide-pools during low tide at SB, brought to the laboratory and exposed on the following day. Contaminants tested were water-accommodated fraction of #4 fuel-oil and benzo[a]pyrene (Sigma). Urchins were exposed individually in 1 L-glass-flasks with 0.8 L of experimental media, for 96 h. Salinity and temperature were maintained at 33–34% and 20 ± 1 °C, respectively. O2 concentration was maintained above 80% saturation. The values of pH varied between 8.0 and 8.3. Physico-chemical parameters were monitored daily. Flasks were aerated throughout the experiment with bubbling compressed air. Test medium was renewed at 48 h. At the end of the test (96 h), ambulacral podia and proximal intestine from each animal were isolated and prepared for ChE and GSTs activity determinations, respectively. Samples were coded and frozen at −80 °C until biochemical assays.Fuel-oil was kindly provided by the oil refinery, PETROGAL, the Portuguese national fuel company. Elutriate of #4 fuel-oil was prepared in a conic flask, by mixing with a magnet (180 rpm) a mixture of 100 g of fuel-oil per litre of artificial seawater (Sera®, Premium) for 6 h. The mixture was allowed to rest for 24 h and the water-accommodated fraction (WAF-100%) decanted. Sea-urchins were individually exposed in glass flasks containing 0.8 L of seawater containing 50, 25, 12.5, 6.25, 3.125, 1.563 or 0% of WAF. Seven replicates per treatment were used.
In experiments with BaP, two sub-lethal concentrations of the chemical were tested: 25 and 100 μg L−1. These concentrations were selected according to preliminary acute toxicity tests. Benzo[a]pyrene was dissolved in acetone, to a concentration never greater than 0.01%. Two control groups were used, one with artificial seawater and another with 0.01% acetone. Seven replicates per treatment were used.
Biomonitoring study
Sea-urchins were collected from 6 locations on the Northwest Portuguese coast during 2003 (Fig. 1). Sampling was performed 4 times a year, one in each year season. Twenty animals were collected per sampling site and season, from tidal-pools, during low tides, at the rocky beaches briefly described below. Upon arrival at the laboratory ambulacral podia and a portion of anterior intestine were taken from each animal and stored at −80 °C for ChE and GSTs activity analysis. The tissues were prepared as previously described. | ||
| Fig. 1 Location of the sampling stations along the Northwest Portuguese Atlantic coast. | ||
Statistical analysis
The results concerning enzymatic activities and levels of contaminants were reported as mean ± standard deviation. Nested analysis of variance (ANOVA) was used to interpret data of enzymatic activity of field animals. Season factor (4 levels) was considered nested in location factor (6 levels). Data were log transformed prior to analysis in order to meet ANOVA assumptions. Enzymatic activity data of different seasons, within the same station, were compared based on post-hoc Newman–Keuls test probabilities. Different treatments in bioassays were compared with control groups using one-way ANOVA, followed by Dunnet test. All analyses were performed using the software package Statistica (Statsoft Inc., Tulsa, US), except determination of Km and Vm, which were determined using Hyper32 software package developed at Liverpool University.36 All statements of statistical significance were established at α = 0.05.Results
Choice of the most suitable tissues for ChE and GST determinations
Values of ChE and GST determined in various tissues are presented in Table 1. ChE activity varied from 1.09 ± 0.20 U mg−1 protein in coelomatic fluid to a maximum of 64.97 ± 10.51 U mg−1 protein in Aristotle’s lantern muscles. GSTs activity could only be detected in the intestine. Proximal and medium intestine portions had similar activity, the distal portion having the lowest activity.| Tissue | ChE | GSTs |
|---|---|---|
| ND—Not detected. | ||
| Coelomatic fluid | 1.09 ± 0.20 | ND |
| Coelomatic membrane | 1.50 ± 0.21 | ND |
| Radial canals | 6.12 ± 1.30 | ND |
| Muscles of Aristotle’s Lantern | 64.97 ± 10.51 | ND |
| Ambulacral podia | 47.20 ± 9.22 | ND |
| Whole intestine | 42. 71 ± 8.91 | 111.60 ± 17.15 |
| Proximal | — | 109.41 ± 15.64 |
| Medium | — | 106.46 ± 13.98 |
| Distal | — | 74.32 ± 19.56 |
Cholinesterase characterization
ChE activity followed an apparent Michaelian behaviour when both AcSCh and PrSCh were used as substrate, but not with BuSCh. The highest activity was observed with AcSCh, with a maximum velocity of 79.0 ± 6.5 U mg−1 protein at 2.6 mM. Theoretical Vm for this substrate was 84.79 ± 5.97 U mg−1 protein and Km was 132.9 ± 35.4 μM. An inhibition by excess of AcSCh occurred at concentrations above 2.6 mM. PrSCh values of Vm and Km were 24.87 ± 0.86 U mg−1 protein and 96.6 ± 17.7 μM, respectively. BuSCh conversion rate was residual compared to the other two substrates and did not follow a hyperbolic behaviour over the range of substrate concentrations tested.Eserine sulfate significantly inhibited ChE activity (F6,14 = 71 131; p < 0.05) with almost full inhibition (>98%) at substrate concentrations equal or higher than 6.25 μM. Inhibition by BW284C51 was also significant at all the concentrations tested (F6,14 = 4796.1; p < 0.05), being 60.85 ± 1.85% at 6.25 μM and above 95% at concentrations equal to or higher than 100 μM (Table 2). Significant inhibition of ChE activity by iso-OMPA (F6,14 = 381.2; p < 0.05) was also found, with a maximum of inhibition of 73% at 8 mM.
| Concentration/μM | Eserine | BW284C51 | Concentration/mM | Iso-OMPA |
|---|---|---|---|---|
| 6.25 | 98.64 ± 0.46 | 60.85 ± 1.85 | 0.25 | 15.63 ± 2.96 |
| 12.5 | 98.96 ± 0.02 | 72.46 ± 1.09 | 0.5 | 19.70 ± 1.39 |
| 25 | 99.21 ± 0.40 | 85.08 ± 0.29 | 1 | 32.01 ± 0.56 |
| 50 | 99.64 ± 0.18 | 91.93 ± 0.49 | 2 | 47.35 ± 6.03 |
| 100 | 99.70 ± 0.12 | 95.43 ± 0.47 | 4 | 64.36 ± 0.80 |
| 200 | 99.77 ± 0.04 | 97.56 ± 0.15 | 8 | 73.05 ± 2.63 |
Laboratory bioassays
Exposure to fuel-oil WAF induced significant effects on GST activity of P. lividus (F6,38 = 18.0; p < 0.05), LOEC being 25% of WAF (Fig. 2). GSTs activity at 50 and 25% of WAF was only 61 and 67% of control group activity, respectively. Furthermore, GST inhibition by fuel-oil WAF followed a log concentration-response model, where:| log GSTs = −0.1728 log WAF + 4.8851 (n = 46; r2 = 0.802) |
 | ||
| Fig. 2 Activity of cholinesterase (ChE) and glutathione S-transferases (GST) after 96 h of exposure to different concentrations of water-accommodated fraction of #4 fuel-oil (WAF). WAF concentration is expressed as a proportion of elutriate (100%) concentration. Values are the mean of 7 animals with correspondent standard error bars. 1 U = 1 nmol of substrate hydrolysed per minute. * Statistically significantly different from the control group (p < 0.05). | ||
GST activity was significantly induced after exposure to BaP (F3,24 = 28.82; p < 0.05). At 100 μg L−1 BaP, GST values were significantly different from the control group, corresponding to an activity 46.4% higher. No significant effects on ChE activity were observed after exposure to BaP (Fig. 3). The vehicle used (acetone) had no effects on the parameters studied.
![Cholinesterase (ChE) and glutathione S-transferases (GST) activity after 96 h of exposure to 25 and 100 μg L−1 of benzo[a]pyrene (BaP). Values are the mean of 7 animals with correspondent standard error bars. * Significantly different from the control (p < 0.05). 0(+) corresponds to control + vehicle (acetone). 1 U = 1 nmol of substrate hydrolysed per minute.](/image/article/2005/EM/b414773a/b414773a-f3.gif) | ||
| Fig. 3 Cholinesterase (ChE) and glutathione S-transferases (GST) activity after 96 h of exposure to 25 and 100 μg L−1 of benzo[a]pyrene (BaP). Values are the mean of 7 animals with correspondent standard error bars. * Significantly different from the control (p < 0.05). 0(+) corresponds to control + vehicle (acetone). 1 U = 1 nmol of substrate hydrolysed per minute. | ||
Biomonitoring study
Seasonal ChE and GSTs activity levels measured in P. lividus during the year 2003 in 6 locations of the Northwest Portuguese coast are presented in Fig. 4. GSTs values ranged from a minimum of 79.2 ± 18.0 U mg−1 protein at BN during winter and a maximum of 279.0 ± 48.0 U mg−1 protein at VC in autumn. The highest GSTs activities were observed in the summer and in the autumn and the lowest during the winter and the spring. Analysis of the hypothesis decomposition of the nested ANOVA for GSTs (Fig. 5) indicated that GSTs activity values were highest at VC and SB, the reference stations, and lowest at CM and BN, the stations in the vicinity of the oil refinery (F5,36 = 72.92; p < 0.05).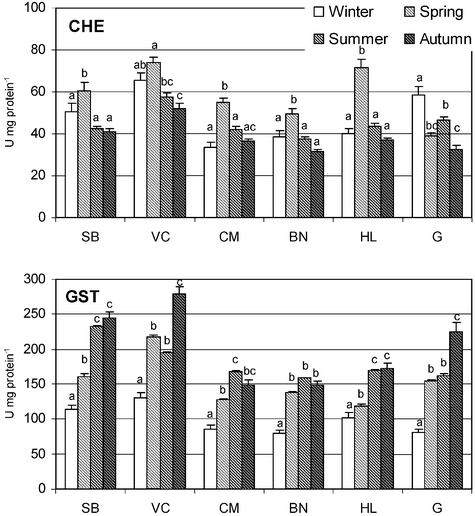 | ||
| Fig. 4 Cholinesterase (ChE) and glutathione S-transferases (GST) activity (U mg protein−1) determined in Paracentrotus lividus from each field station (SB – S. Bartolomeu; VC – Vila Chã; CM – Cabo do Mundo; BN – Boa Nova; HL – Homem do Leme; G – Granja) in winter, spring, summer and autumn. Values are the mean of 20 animals with correspondent standard error bars. Similar letters correspond to similar values of enzymatic activity within the same station, based on post-hoc Newman–Keuls test probabilities (p < 0.05). 1 U = 1 nmol of substrate hydrolysed per minute. | ||
 | ||
| Fig. 5 Cholinesterase (ChE) and glutathione S-transferase (GST) activities (log (U mg−1 protein)) for each field station with the variable seasons nested in location, determined in Paracentrotus lividus from the various stations (SB – S. Bartolomeu; VC – Vila Chã; CM – Cabo do Mundo; BN – Boa Nova; HL – Homem do Leme; G – Granja), after a Type III decomposition of the hypothesis. Values are the mean of 20 animals with correspondent 95% confidence limits. Similar letters correspond to stations with similar enzymatic activity values based on post-hoc Newman–Keuls test probabilities (p < 0.05). Vertical bars denote 95% confidence intervals. | ||
ChE values ranged from a minimum of 31.7 ± 4.5 U mg−1 protein at BN during autumn and a maximum of 71.8 ± 13.3 U mg−1 protein at VC in spring. In general, the highest ChE activities were observed during spring (Fig. 4). Hypothesis decomposition of the nested ANOVA for ChE (Fig. 5) showed that ChE values were highest at VC, one of the reference stations and lowest at BN, the closest station to the oil refinery (F5,366 = 55.01; p < 0.05).
Discussion
One of the objectives of this study was to choose the most suitable tissue for measuring enzymatic activities, taking into consideration the enzymatic level of each tissue, ethical issues (destructive/non-destructive analysis) and type of exposure to environmental seawater. From the tissues tested, perioral ambulacra were chosen for further ChE assays because they have high activity (47.20 ± 9.22 U mg−1 protein), sampling is non-destructive and these organs are in direct contact with surrounding seawater. We could not find ChE activity values for P. lividus or other Echinozoa in the literature to compare with our data. ChE values of P. lividus are considerably higher than those determined in A. rubens from the North Sea.37 Nevertheless, the values are not directly comparable, since A. rubens ChE activity was measured in the pyloric caeca, while in P. lividus it was measured in ambulacra, an organ with sensorial functions among others (e.g. locomotion, respiration, excretion), apart from differences of temperature, species habitat and phylogeny. Ambulacra collection is non-destructive, allowing repeated measurements in the same individuals, and/or sample collection and release of the specimen alive. Besides, these organs are in direct contact with environmental water, which makes them an organ with ideal characteristics to sample for ecotoxicological purposes.Intestine was the only tissue tested where GSTs activity could be detected, with the technique and substrate used. Proximal intestine had the highest enzymatic activity (109.41 ± 15.64 U mg−1 protein), it is an easy section to locate and dissect, and it is more prone than other sections to be void of food or faeces that may contaminate samples. Therefore, proximal intestine was used in all further GST determinations.
The characterization of P. lividus ambulacra ChE was the second step of the study. Almost full inhibition of enzymatic activity by eserine sulfate at concentrations in the range of 10 μM was found. This is a characteristic of ChE,38 therefore, this result indicates that the measured activity is mainly due to ChE and not to other esterases. AcSCh was the substrate with the highest conversion rate, although PrSCh was also hydrolysed at a considerable rate. BuSCh was only hydrolysed at vestigial rates. Furthermore, ChE activity was inhibited by an excess of AcSCh and almost fully inhibited by BW284C51, which are properties of mammalian AChE. However, like mammalian PChE, P. lividus ChE was sensitive to ISO-OMPA. Therefore, the results of this study suggest the presence of a ChE in P. lividus ambulacra showing characteristics of both AChE and PChE. The most adequate substrate for ChE determinations in this species and tissue is AcSCh.
In the laboratory bioassays, WAF of fuel-oil significantly inhibited GST activity of sea-urchins. This result is in contradiction with the effects of fuel-oil exposure on GST of other marine invertebrates observed in experiments performed in our laboratory. In these studies, induction of GST activity was observed in Mytilus galloprovincialis after laboratory exposure to fuel-oil from the “Coral Bulker” spill,7 and in Mytilus galloprovincialis39 and Crangon crangon40 exposed to #4 fuel-oil WAF. These results suggest that the effects of fuel-oils on P. lividus GSTs are different from those induced on mussels and shrimps GSTs. No effects of #4 fuel-oil WAF on P. lividus ChE activity were observed at the concentrations tested, in good agreement with the results obtained in Mytilus galloprovincialis39 and Crangon crangon40 exposed to the same product. On the contrary, Mytilus galloprovincialis exposed to fuel released from “Coral Bulker” presented a significant inhibition of ChE,7 indicating that different fuels have distinct effects on ChE activity and, suggesting that the presence and/or concentration of anticholinesterase agents changes in the different mixtures.
The induction of P. lividus GST activity after exposure to BaP, a polycyclic aromatic hydrocarbon (PAH) usually present in petroleum-derived products, is in good agreement with the findings reported in the literature for different mussel species.16,40,41 However, it contrasts with other studies, also performed in mussels, where unchanged activity 16,42 and down-regulation43,44 were found. No effects on ChE activity were observed on animals exposed in laboratorial conditions to BaP. Also, exposure of Eisenia fetida to BaP contaminated soils had no effect on ChE activity.45 On the contrary, inhibition of ChE activity was observed in mussel gills after BaP exposure.41
A considerable seasonal variation in both GST and ChE activities was found in all the sampling sites. The highest ChE values were observed in spring and the lowest in autumn. These results are in good agreement with those reported for mussels from the same coastal zone46. The maximal GST activity was observed in summer and autumn, while the minimal was determined during the winter. In P. maximus, from Brest bay, France, the maximal GST activity was also observed in the summer,47 as well as for Mytilus edulis in Ireland,48M. galloprovincialis in Italy and South Morocco17,49 and, Perna perna in South Morocco.49 Unfortunately, we could not find echinoderms’ GST values in the literature to compare our results.
In the biomonitoring study, GST activity was significantly inhibited in animals collected in the contaminated sites (CM, BN, HL and GR) relative to the reference sampling stations (SB and VC). CM and BN, which are close to an oil refinery and to Leixões harbour and contaminated with petroleum-derived products,31–33 displayed the lowest values (Fig. 5). This seems to be in good agreement with the GST inhibition found in animals exposed to #4 fuel-oil in the laboratorial bioassays performed in the present study. However, this is in contradiction with the results obtained in mussels after the “Aegean Sea”50 and “West Falmouth”43 oil spills, where no alterations in GST were found.
A significant inhibition of ChE activity was also observed in mussels from contaminated sites (CM, BN, HL and GR) relative to animals from VC, one of the reference sites. This is in good agreement with the results that have been found in mussels collected in the same sites. Interestingly, the lowest activities were found in BN and CB, the sites in the neighbourhood of the harbour and of the oil refinery. The results from the laboratorial bioassays with animals exposed to #4 fuel-oil WAF apparently did not support a possible decrease of ChE activity due to petroleum-derived products, contrary to what has been found for M. galloprovincialis.7,46 The differences between field and laboratorial assays, may be due at least to two reasons: first, the composition of the fuel tested in the laboratory is considerably different from the products found in the field, the former having a low concentration of anticholinesterase agents; and, second, the acute exposure (96 h) is not sufficient to induce the inhibition of P. lividus ChE. However, significant differences between animals collected in the two reference sampling stations were found, with sea-urchins from SB showing a reduced activity relative to those collected in Vila-Chã. Unfortunately, our experimental design does not allow us to go deeply into this question, further studies should be undertaken to ascertain the suitability of SB as a reference site in future experimental designs.
In conclusion, the results of this study indicate that: (i) ambulacra and the anterior portion of the intestine are the most suitable tissues for measuring ChE and GST activity, respectively, in P. lividus; (ii) the ChE present in the ambulacra is an atypical ChE showing properties of both AChE and PChE; (iii) P. lividus GST is inhibited by #4 fuel-oil WAF oil and induced by BaP, while ChE is not sensitive to any of these xenobiotics, after an acute exposure (96 h), and (iv) in spite of the significant seasonal variations observed in both GST and ChE activity of wild P. lividus populations, these enzymes were capable of discriminating sites with different contamination levels and, thus, they are suitable for use as biomarkers in biomonitoring studies in the coastal zone.
Acknowledgements
Isabel Cunha was supported by a post-doctoral fellowship (BPD/5691/2001) from the Foundation for Science and Technology (FCT) – Portugal. This study was also supported by FCT through the project CONTROL (PDCTM/PP/MAR/15266/1999) and by FEDER funds. The participation of Luz Maria García de la Parra in this study was supported by a bilateral cooperation project between Portugal (GRICES and Instituto Camões) and Mexico (CONACYT). The authors would like to thank Dr Jorge Ribeiro and “Petrogal” (Portugal) for kindly providing the #4 fuel-oil used in this study.References
- P. den Besten, S. Valk, E. van Weerlee, R. Nolting, J. Postma and J. Everaarts, Mar. Environ. Res., 2001, 51(4), 365–387 CrossRef CAS.
- J. McHenery, G. Linley-Adams, D. Moore, G. Rodger and I. Davies, Aquat. Toxicol., 1997, 38(1–3), 125–143 CrossRef CAS.
- R. Owen, L. Buxton, S. Sarkis, M. Toaspern, A. Knap and M. Depledge, Mar. Pollut. Bull., 2002, 44(10), 1010–1017 CrossRef CAS.
- A. Lundebye, T. Curtis, J. Braven and M. Depledge, Aquat. Toxicol., 1997, 40(1), 23–36 CrossRef CAS.
- A. Hamza-Chaffai, M. Romeo, M. Gnassia-Barelli and A. El Abed, Bull. Environ. Contam. Toxicol., 1998, 61, 397–404 CrossRef CAS.
- R. Brown, T. Galloway, D. Lowe, A. Browne, A. Dissanayake, M. Jones and M. Depledge, Aquat. Toxicol., 2004, 6(3), 267–278 CrossRef.
- S. M. Moreira, M. Moreira-Santos, R. Riberio and L. Guilhermino, Ecotoxicology, 2004, 13(7), 619–630 CrossRef CAS.
- P. Mora, D. Fournier and J.-F. Narbonne, Comp. Biochem. Physiol., Part C, 1999, 122(3), 353–361 CrossRef CAS.
- P. Key and M. Fulton, Pestic. Biochem. Physiol., 2002, 72(3), 186–192 CrossRef CAS.
- D. R. Buhler and D. E. Williams, Aquat. Toxicol., 1998, 11(1–2), 19–28.
- Y. S. Zhang, T. Anderson and L. Förlin, Comp. Biochem. Physiol., Part B, 1990, 95, 247–253 Search PubMed.
- R. Pinkus, S. Bergelson and V. Daniel, Biochem. J., 1993, 290, 637–640 CAS.
- X. Stien, P. Percic, M. Gnassia-Barelli, M. Roméo and M. Lafaurie, Environ. Pollut., 1998, 99(3), 339–345 CrossRef CAS.
- I. Boutet, A. Tanguy and D. Moraga, Gene, 2004, 329, 147–157 CrossRef CAS.
- B. Gowland, A. McIntosh, I. Davies, C. Moffat and L. Webster, Mar. Environ. Res., 2002, 54(3–5), 231–235 CrossRef CAS.
- C. Cheung, G. Zheng, A. Li, B. Richardson and P. Lam, Aquat. Toxicol., 2001, 52, 189–203 CrossRef CAS.
- F. Regoli and G. Principato, Aquat. Toxicol., 1995, 31(2), 143–164 CrossRef CAS.
- M. H. Depledge and A. K. Lundebye, Comp. Biochem. Physiol., Part C, 1996, 113(2), 277–282 CrossRef.
- C. Nasci, N. Nesto, R. A. Monteduro and L. Da Ros, Mar. Environ. Res., 2002, 54, 811–816 CrossRef CAS.
- A. H. Ringwood, J. Hoguet and C. J. Keppler, Mar. Environ. Res., 2002, 54, 793–797 CrossRef CAS.
- C. Ridder and J. Lawrence, in Echinoderm Nutrition, ed. M. Jangoux and J. M. Lawrence, A. A. Balkema Publishers, Rotterdam, The Netherlands, 1982, pp. 57–115 Search PubMed.
- J. Valentine and K. Heck, J. Exp. Mar. Biol. Ecol., 1991, 154, 215–230 CrossRef.
- N. Andrew, Ecology, 1993, 74, 292–302 Search PubMed.
- H. Leinaas and H. Christie, Oecologia, 1996, 105, 524–536 CrossRef.
- L. Benedetti-Cecchi and F. Cinelli, Mar. Ecol. Prog. Ser., 1995, 126, 203–212 CrossRef.
- P. Hayward and J. Ryland, in The Marine Fauna of the British Isles and North-West Europe, Clarendon Press, Oxford, 1990, vol. 2 Search PubMed.
- G. Ellman, K. Courtney, V. Andres Jr. and R. Featherstone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS.
- L. M. García, B. Castro, R. Ribeiro and L. Guilhermino, Biomarkers, 2000, 5(4), 274–284 CrossRef CAS.
- W. Habig, M. Pabst and W. Jakoby, J. Biol. Chem., 1974, 249(22), 7130–7139 CAS.
- G. N. Wilkinson, Biochem. J., 1961, 80, 324–332 CAS.
- L. Serra, MSc Thesis, University of Porto, Portugal, 1998.
- M. A. Salgado and L. Serra, in Proceedings of First International Congress on Petroleum Contaminated Soils, Sediments, and Water Analysis, Assessment and Remediation, London, 2001 Search PubMed.
- M. C. F. Leal, M. T. Vasconcelos, I. Sousa-Pinto and J. P. S. Cabral, Mar. Pollut. Bull., 1997, 34, 1006–1015 CrossRef CAS.
- J. M. A. Dias, R. Gonzalez, C. Garcia and V. Diaz-del-Rio, Prog. Oceanogr., 2002, 52, 249–259 CrossRef.
- N. Guerreiro and P. B. Pereira, Poluição e qualidade da água, Instituto da Água, Lisbon, Portugal, 2002 Search PubMed.
- http://homepage.ntlworld.com/john.easterby/hyper32.html .
- P. den Besten, S. Valk, E. van Weerlee, R. Nolting, J. Postma and J. Everaarts, Mar. Environ. Res., 2001, 51(4), 365–387 CrossRef CAS.
- M. Eto, Organophosphorous pesticides: Organic and Biochemical Chemistry, CRC Press, Cleveland, Ohio, 1974 Search PubMed.
- I. Lima and L. Guilhermino, 8° Encontro Nacional de Ecologia, Évora, Portugal, 2003 Search PubMed.
- S. Meneses, A. M. V. M. Soares, L. Guilhermino and M. R. Peck, 14th Annual Meeting of Setac Europe, Prague, Czech Republic, 2004 Search PubMed.
- D. Sheehan, K. Crimmins and G. Burnell, in Bioindicators and Environmental Management, ed. D. W. Jeffrey and B. Madden, Academic Press, London, 1991, pp. 419–425 Search PubMed.
- F. Akcha, C. Izuel, P. Venier, H. Budzinski, T. Burgeot and J.-F. Narbonne, Aquat. Toxicol., 2000, 49, 269–287 CrossRef CAS.
- J. Teal, J. Farrington, K. Bums, J. Stegeman, B. Tripp, B. Woodin and C. Phinney, Mar. Pollut. Bull., 1992, 24, 607–614 CrossRef CAS.
- X. R. Michel, P. Suteau, L. W. Robertson and J. F. Narbonne, Aquat. Toxicol., 1993, 27, 335–344 CrossRef CAS.
- M. Saint-Denis, J. F. Narbonne, C. Arnaud, E. Thybaud and D. Ribera, Soil Biol. Biochem., 1999, 31(131), 1837–1846 CrossRef CAS.
- S. M. Moreira and L. Guilhermino, Environ. Monit. Assess., 2005, 105 Search PubMed , in press.
- G. Le Pennec and M. Le Pennec, Aquat. Toxicol., 2003, 64, 131–142 CrossRef CAS.
- A. Power and D. Sheehan, Comp. Biochem. Physiol., Part C, 1996, 114, 99–103 CrossRef.
- A. Kaaya, S. Najimi, D. Ribera, J. F. Narbonne and A. Moukrim, Bull. Environ. Contam. Toxicol., 1999, 62, 623–629 CrossRef CAS.
- M. Solé, C. Porte, X. Biosca, C. Mitchelmore, J. Chipman, D. Livingstone and J. Albaigés, Comp. Biochem. Physiol., Part C, 1996, 113(2), 257–265 CrossRef.
| This journal is © The Royal Society of Chemistry 2005 |
