Self-assembly of coordination molecular baskets as inorganic analogues of cyclotriveratrylenes (CTV)†
Sheng-Hui
Li
a,
Hai-Ping
Huang
a,
Shu-Yan
Yu
*abe,
Yi-Zhi
Li
*c,
Hui
Huang
a,
Yoshihisa
Sei
d and
Kentaro
Yamaguchi
d
aState Key Laboratory of Polymer Physics and Chemistry, Institute of Chemistry, and Graduate School of the Chinese Academy of Sciences, The Chinese Academy of Sciences, Beijing, 100080, China. E-mail: syu@iccas.ac.cn; Fax: (+86)10-6255-9373; Tel: (+86)10-6256-4823
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, China
cState Key Laboratory of Coordination Chemistry, Institute of Coordination Chemistry, Nanjing University, Nanjing, 210093, China
dLaboratory of Analytical Chemistry, Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences at Kagawa Campus, Tokushima Bunri University, Shido, Sanuki-city, Kagawa 769-2193, Japan
eState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, 730000, China
First published on 1st June 2005
Abstract
A [3 + 3] modular self-assembly gives rise to the formation of basket-shaped, crown ether-functionalized, nano-sized trimetallo-macrocycles, which function as structural analogues of cyclotriveratrylenes (CTV).
The construction of container molecules remains an interesting topic in supramolecular chemistry, particularly bowl-shaped molecules, such as calixarenes and cyclotriveratrylenes (CTV), because of their potential applications in molecular encapsulation, catalysis, assembly and self-assembly, etc.1 Over the last decade, numerous well-defined metal–organic container molecules with high symmetries (regular polygons and polyhedra such as molecular squares, molecular grids, tetrahedronal cages, etc.) have been established by metal-directed molecular self-assembly.2 However, only a few examples with low-symmetries (irregular assemblies)2c like bowl-shaped metallo-organic entities have been reported, which have attracted particular attention because they are capable of behaving as structural analogues of calixarenes or CTV.3–6 Recently, we have succeeded in the self-assembly of such irregular metallo-organic container molecules with calixarene features, such as molecular bowls, crowns and capsules.7,8 It should be noted that we have developed a cavity-tunable modular self-assembly approach,7,8a which has provided a scafford for design and architecture of structurally and functionally new metallo-organic container molecules. In order to enlarge the cavity and to modify the functionality of such metal–organic frameworks, herein we introduced the 1,10-phenanthroline-crown ethers ligand9 coordinated Pd(II) and Pt(II) complexes as building blocks to self-assemble with 4,7-phenanthroline (L) into basket-like container molecules as shown in Scheme 1.
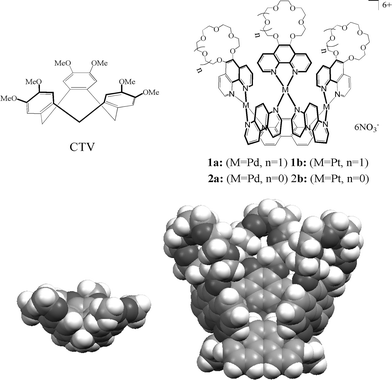 | ||
| Scheme 1 CTV and its inorganic analogues: self-assembling molecular baskets (the CPK diagrams calculated using the CAChe 6.1.1 program10). | ||
The treatment of [Pd(NO3)2(phen-crown-6)] with a solution containing one equivalent of L in D2O at room temperature over several minutes leads to formation of [{Pd(phen-crown-6)}3L3](NO3)6 (1a) in quantitative yield. The 1H NMR spectrum shown in Fig. 1 revealed the presence in solution of a symmetrical structure and is consistent with a single product. Integration of the signals indicates a 1 : 1 ratio of metal-coordination-complex fragment to L, and this is supported by elemental analyses. The resonance signals labelled Δ correspond to the ligand L. The Pt analogue, [{Pt(phen-crown-6)}3L3](NO3)6 (1b) was obtained at elevated temperature (100 °C, 36 h) by a similar route to that of 1a, and the spectra of 1b are similar to those of 1a. Therefore, 1b possesses the same structure as 1a, that is, a trimetal-centre molecular basket with a charge of 6+.
 | ||
| Fig. 1 Self-assembly of 1a and 1b. a) 1H NMR spectrum of 1a, b) 1H NMR spectrum of 1b. | ||
The formation of the proposed molecular basket 1a (and 1b) was further supported by cold spray ionization mass spectrometry (CSI-MS)11: [1a − 2NO3−]2+ (1175.6), [1a − 3NO3−]3+ (763.1), [1b − 2NO3−]2+ (1308.4) and [1b − 4NO3−]4+ (623.2).
A single crystal of 1a was obtained by slow evaporation of its aqueous solution. The X-ray crystallographic analysis‡ confirms the molecular basket structure of 1a (Fig. 2). It is interesting that the two kinds of modules make up two different cavities, one is hydrophobic, while the other is hydrophilic due to the crown ether modified rims. The three Pd atoms lie on a nearly perfect equilateral triangle with Pd⋯Pd distances of 7.65(1), 7.54(7) and 7.64(3) Å. The three rigid modules of L make up the lower cavity (below the tri-Pd triangle) exhibiting hydrophobic character and they are bridged by three square-planar coordination modules [Pd(phen-crown-6)] in a syn, syn, syn orientation. The dimensions of the resultant bowl are as follows: (8.40(1), 8.34(3) and 8.38(2) Å in rim lengths (i.e. the distances between the midpoint of the 1,10 position of L); 3.6(2) Å in depth; dihedral angles between L and the tri-Pd triangular plane: 52.9, 49.6, 54.5°.
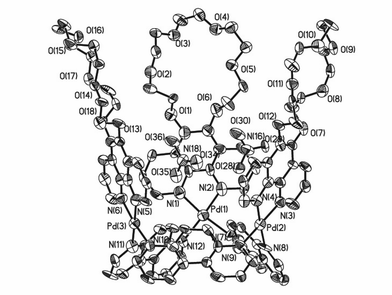 | ||
| Fig. 2 ORTEP drawing of 1a encapsulating two NO3− anions within the upper cavity (50% probability ellipsolids. The free counter anions as well as solvent molecules and hydrogen atoms have been removed for clarity). | ||
The upper cavity (above the tri-Pd triangle consisting of the three square planar Pd(phen-crown-6) modules) has a cavity size as follows: 11.69(2), 11.78(1) and 16.17(2) Å in rim lengths; 12.74(3) Å in depth; dihedral angles between the square planar modules and the tri-Pd triangular plane: 81.4, 73.6 and 77.2°. The upper cavity, which possess the more open and larger and deeper void space than the lower cavity, is expected to exhibit more important receptor chemistry. Remarkably, two nitrate anions (N16–O28–O29–O30 and N18–O34–O35–O36) are encapsulated within the upper hydrophilic cavity of the basket. On the contrary, there are no nitrates located inside the lower cavity, a similar phenomenon was previously observed in the case of a molecular crown,8a due to the hydrophobic properties of the aromatic module constructed cavities.
By analogy, starting from [Pd(NO3)2(phen-crown-5)] or [Pt(NO3)2(phen-crown-5)] and L, a similar self-assembly was successfully achieved which was confirmed by 1H NMR, and CSI-MS spectroscopy as well as molecular modeling. In the CSI-MS spectra for 2a in methanol, the peaks appearing at 719.0 and 1109.5 can be assigned to [2a − 3NO3−]3+, [2a − 2NO3−]2+, respectively. Similarly, in the CSI-MS spectra for 2b in methanol, the peaks presenting at 459.8, 590.2, 807.6 and 1242.4 are attributed to [2b − 5NO3−]5+, [2b − 4NO3−]4+, [2b − 3NO3−]3+, and [2b − 2NO3−]2+, respectively.
The ability of such a CTV-linked container molecule to include aromatic carboxylate anions12 (such as benzoic acid sodium salt, p-toluic acid sodium salt and p-anisic acid sodium salt) in its cavity has been studied by means of 1H NMR spectroscopy. A significant upfield shift was observed for the aromatic protons of the anion of benzoic acid in D2O upon complexation with an equimolecular amount of 1a (Δδ = −0.24, −0.30 and −0.42 for H2, H3 and H4). However, compared with the anion receptor chemistry exhibited by [{Pd(en)}3L3](NO3)6,8a1a does not show important interactions with SO42− in aqueous solution. In fact, the 1H NMR monitoring experiments showed that the addition of an excess quantity of SO42− to a solution of 1a does not lead to any significant chemical shift. Additionally, the formation of 1 : 1 host–guest complex [Co(C2B9H11)2]− to 1a was tentatively studied by NMR titration.
In summary, we have described self-assembling molecular baskets with novel crown-ether functionalized nano-cavities. Further investigation will be directed toward their potentials as sensors for binding large cluster anions (such as cobalticarborane, polyoxometalate anions, etc.) or for multiple recognition of cations and anions.
This project was supported by the National Natural Science Foundation of China (No. 90206013, No. 50373051) and the Chinese Academy of Sciences (One-Hundred Young Talents).
References
- (a) J.-M. Lehn, Supramolecular Chemistry, Concepts and Perspectives, VCH, Weinheim, 1995 Search PubMed; (b) D. J. Cram and J. M. Cram, Container Molecules and Their Guests, The Royal Society of Chemistry, Cambridge, UK, 1994 Search PubMed.
- (a) C. J. Jones, Chem. Soc. Rev., 1998, 27, 289 RSC; (b) M. Fujita, Chem. Soc. Rev., 1998, 27, 417 RSC; (c) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS; (d) M. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa and K. Birada, Chem. Commun., 2001, 509 RSC; (e) F. A. Cotton, C. Lin and C. A. Murillo, Acc. Chem. Res., 2001, 34, 759 CrossRef CAS; (f) P. H. Dinolfo and J. T. Hupp, Chem. Mater., 2001, 13, 3113 CrossRef CAS; (g) J. A. R. Navarro and B. Lippert, Coord. Chem. Rev., 2001, 222, 219 CrossRef CAS.
- (a) R.-D. Schnebeck, E. Freisinger and B. Lippert, Angew. Chem. Int. Ed., 1999, 38, 168 CrossRef CAS; (b) R.-D. Schnebeck, E. Freisinger, F. Glahe and B. Lippert, J. Am. Chem. Soc., 2000, 122, 1381 CrossRef CAS.
- (a) H. Rauter, E. C. Hillgeris and B. Lippert, Chem. Commun., 1992, 1385 RSC; (b) H. Rauter, E. C. Hillgeris and B. Lippert, J. Am. Chem. Soc., 1994, 116, 616 CrossRef CAS; (c) J. A. R. Navarro, M. B. L. Janik, E. Freisinger and B. Lippert, Inorg. Chem., 1999, 38, 426 CrossRef CAS; (d) J. A. R. Navarro, E. Freisinger and B. Lippert, Inorg. Chem., 2000, 39, 2301 CrossRef CAS; (e) E. Barea, J. A. R. Navarro, J. M. Salas, M. Willermann and B. Lippert, Chem. Eur. J., 2003, 9, 4414 CrossRef CAS; (f) M. A. Galindo, J. A. R. Navarro, M. A. Romero and M. Quiros, Dalton Trans., 2004, 1563 RSC; (g) J. A. R. Navarro and J. M. Salas, Chem. Commun., 2000, 235 RSC.
- (a) H. Chen, M. M. Olmstead, D. P. Smith, M. F. Maestre and R. H. Fish, Angew. Chem. Int. Ed., 1995, 34, 1514 CAS; (b) H. Piotrowski, G. Hilt and K. Severin, J. Am. Chem. Soc., 2001, 123, 2699 CrossRef CAS; (c) M.-L. Lehaire, R. Scopelliti, H. Piotrowski and K. Severin, Angew. Chem. Int. Ed., 2002, 41, 1419 CrossRef CAS.
- S.-W. Lai, M. C.-W. Chan, S.-M. Peng and C.-M. Che, Angew. Chem. Int. Ed., 1999, 38, 669 CrossRef CAS.
- S.-Y. Yu, S.-H. Li, H.-P. Huang, Z.-X. Zhang, Q. Jiao, H. Shen, X.-X. Hu and H. Huang, Curr. Org. Chem., 2005, 9, 555 CrossRef CAS and references cited therein.
- (a) S.-Y. Yu, H. Huang, H.-B. Liu, Z.-N. Chen, R. Zhang and M. Fujita, Angew. Chem. Int. Ed., 2003, 42, 686 CrossRef CAS; (b) F. A. Cotton, L. Peng, C. Lin, C. A. Murillo, X. Wang, S.-Y. Yu and Z.-X. Zhang, J. Am. Chem. Soc., 2004, 126, 1518 CrossRef CAS; (c) S.-Y. Yu, T. Kusukawa, K. Biradha and M. Fujita, J. Am. Chem. Soc., 2000, 122, 2665 CrossRef CAS; (d) M. Fujita, S.-Y. Yu, T. Kusukawa, H. Funaki, K. Ogura and K. Yamaguchi, Angew. Chem. Int. Ed., 1998, 37, 2082 CrossRef CAS.
- (a) C. J. Pederson, J. Am. Chem. Soc., 1967, 89, 2495 CrossRef CAS; (b) B. Deitrich, J.-M. Lehn and J. P. Sauvage, Tetrahedron Lett., 1969, 2885 CrossRef CAS; (c) C. R. Rice, A. Guerrero, A. R. Bell, R. L. Paul, G. R. Motson, J. C. Jeffery and M. D. Ward, New J. Chem., 2001, 25, 185 RSC.
- CAChe 6.1.1 for Windows, Fujitsu Ltd., Chiba, Japan, 2003 Search PubMed.
- K. Yamaguchi, J. Mass Spectrom., 2003, 38, 473 CrossRef CAS.
- (a) A. Bianchi, K. Bowman-James and E. Garcia-Espaňa, Supramolecular Chemistry of Anions, Wiley, New York, 1997 Search PubMed; (b) P. A. Gale, Coord. Chem. Rev., 2000, 199, 181 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section; 1H NMR spectra of 1a, 1b, 2a, 2b; CSI-MS spectra of 1a, 1b, 2a, 2b; crystallography: experimental and refinement. See http://www.rsc.org/suppdata/dt/b5/b504639d/ |
‡ Crystal data for 1a: C102H114N18O42Pd3, M
= 2583.31; triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 17.182(3), b
= 17.856(4), c
= 23.319(5)
Å, α
= 103.37(3), β
= 91.54(3), γ
= 105.97(3)°, V
= 6660(3)
Å3, Dc
= 1.288 g cm−3. Z
= 2, F(000)
= 2652, μ(Mo-Kα)
= 0.482 mm−1, λ
= 0.71073 Å, 2θmax
= 52°, 47324 reflections measured, 18658 observed reflections (I > 2σ(I)). Number of parameters 1585, R1
= 0.0558, wR2
= 0.1159, GOF = 1.03. CCDC reference number 267395. See http://www.rsc.org/suppdata/dt/b5/b504639d/ for crystallographic data in CIF or other electronic format. , a
= 17.182(3), b
= 17.856(4), c
= 23.319(5)
Å, α
= 103.37(3), β
= 91.54(3), γ
= 105.97(3)°, V
= 6660(3)
Å3, Dc
= 1.288 g cm−3. Z
= 2, F(000)
= 2652, μ(Mo-Kα)
= 0.482 mm−1, λ
= 0.71073 Å, 2θmax
= 52°, 47324 reflections measured, 18658 observed reflections (I > 2σ(I)). Number of parameters 1585, R1
= 0.0558, wR2
= 0.1159, GOF = 1.03. CCDC reference number 267395. See http://www.rsc.org/suppdata/dt/b5/b504639d/ for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |
