Probing segmental order in lipid bilayers at variable hydration levels by amplitude- and phase-modulated cross-polarization NMR†
Sergey V.
Dvinskikh
*ab,
Vasco
Castro
a and
Dick
Sandström
*a
aDivision of Physical Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91, Stockholm, Sweden. E-mail: sergey@physc.su.se; dick.sandstrom@physc.su.se; Fax: +46 8 152187; Tel: +46 8 161260
bInstitute of Physics, St. Petersburg State University, 198504, St. Petersburg, Russia. Fax: +7 812 4287240; Tel: +7 812 4287559
First published on 12th August 2005
Abstract
The conformational response of dimyristoylphosphatidylcholine bilayers in the liquid crystalline phase to hydration is investigated by a novel magic-angle spinning cross-polarization NMR technique.
The investigation of liquid crystalline systems such as fluid phase lipids has been greatly advanced by solid-state NMR techniques probing anisotropic spin interactions. Deuterium NMR has in the past been the most popular approach for characterizing these systems,1 but has the disadvantage that site-specific isotopic labelling is required. Furthermore, the resonance assignment and resolution of spectra produced by multiply 2H-labelled molecules are problematic. Therefore, there has been a growing interest in measuring one-bond 1H–13C dipolar couplings since the dipolar tensor provides information similar to the 2H electric field gradient tensor.2 Highly resolved 13C spectra can be recorded at natural abundance and may be assigned by routine NMR methods.3 The problem of signal overlap in dipolar spectra of complex chemical systems is solved by using two-dimensional (2-D) separated local field (SLF) spectroscopy.4 These experiments can be carried out under magic-angle spinning (MAS) conditions, thus avoiding tedious preparation of a macroscopically oriented sample (such as layering the membranes on thin glass plates),5,6 and problems of low mechanical sample stability and poor signal intensity.6 While fast MAS suppresses the dipolar couplings, it is possible to combine rapid sample rotation with radio-frequency (rf) pulse schemes to recouple the informative 1H–13C interactions.7 Many of the previously employed SLF sequences for investigating lipid membranes provide limited resolution of the heteronuclear spin–spin interactions (considerably lower than 2H data),2 due to small dipolar scaling factors,2a,2b limited spectral range,2b curve-fitting errors,2c and unfavorable relaxation behaviour. Moreover, some techniques are insensitive to small couplings,2c while others require specialized hardware.2a Recently, some of us reported an efficient SLF method8a based on symmetry based recoupling which, unfortunately, has also a rather small heteronuclear dipolar scaling factor.
In this communication, we introduce a novel NMR recoupling scheme which is efficient in soft materials and provides unparalleled dipolar resolution in fluid phase lipids. The technique is demonstrated on a model membrane sample, and is used to study the relationship between hydration and segmental order of the lipid molecules.
The new SLF experiment is based on recent advances in the design of recoupling sequences made in our laboratory8 and uses on-resonance amplitude- and phase-modulated (APM) cross-polarization (CP) rf pulses during the evolution period t1 (see Fig. 1a). In contrast to previous phase-modulated9a,9b or phase- and amplitude-modulated9c,9d CP methods, our approach does not rely on synchronization of the rf pulses with the sample rotation, and the modulation is fast on the time scale of the sample rotation period. The flip angles of the 1H rf pulses correspond to small multiples of 180° rotations. Efficient 1H–13C dipolar recoupling is achieved by an alternation of the Hartmann–Hahn matching condition between the +1 and −1 sidebands.8c The lowest order average dipolar Hamiltonian is, for a two spin system, given by
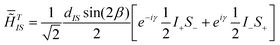 | (1) |
 | ||
| Fig. 1 (a) APM-CP pulse sequence for 2-D SLF spectroscopy. After CP signal enhancement, the dipolar evolution period is initiated by inverting the phase of the 1H spin-lock field. The CP fields during t1 are phase- and amplitude-modulated as explained in the text. Finally, the 13C signal is detected in the presence of heteronuclear 1H decoupling. (b) Selected 1H-13C dipolar cross-sections through a 2-D APM-CP spectrum of unoriented DMPC obtained at 41 °C and nw ≈ 8 (see Fig. 2 for the chemical structure). The 1-D 13C isotropic chemical shift spectrum is displayed to the left. A detailed discussion of the dipolar couplings in hydrated DMPC can be found elsewhere.8d The experiment was carried out on a Chemagnetics Infinity-400 spectrometer at a spinning frequency of 8 kHz using a 4 mm rotor. The mass of the sample was ca. 35 mg. The 1H field strength during the evolution period was set to γB1,H/2π = 61 kHz, and the t1 increment time of 32.3 μs corresponded to two 360° phase-alternated CP pulses. Thiry-two transients were recorded for each of the 100 t1 increments (total acquisition time of 4 h). | ||
Selected 1H-13C cross-sections extracted from a 2-D APM-CP spectrum of dimyristoylphosphatidylcholine (DMPC) in the liquid crystalline Lα phase are shown in Fig. 1b. A comparison of these spectra with those previously obtained for phospholipids by other SLF methods2 indicates that the APM-CP sequence results in superior dipolar resolution. The majority of the CH2 groups in DMPC produce only one splitting. The slice through the C2 peak, however, contains two superimposed powder patterns showing that the two fatty acyl chains behave differently.8d The same conclusion can be drawn from the C3, C12, and C13 signals. (While the C12 and C13 signals are unresolved in the direct dimension, the presence of two distinct powder patterns is apparent in 2-D plots.8d) The spectral splitting displayed by a CH group is given by 2πΔνCH = (1/√2)SCHbCH, where SCH is the segmental order parameter and bCH is the one-bond rigid lattice dipolar coupling (here assumed to be bCH/2π = 21.5 kHz). A CH2 segment containing two identical 1H–13C interactions exhibits a splitting of 2πΔνCH2 = SCHbCH (see the electronic supplementary information).†
To study the structural response of DMPC to hydration, we have carried out APM-CP experiments as a function of the number of water molecules per lipid (nw) over the range 4 ≤ nw ≤ 50. As judged by the appearance of the 1H and 13C MAS spectra (not shown), all measurements were conducted in the Lα phase. Since the dipolar couplings for all C–H bond segments are obtained in a single experiment, identical hydration level is ensured when comparing the various molecular fragments.
Fig. 2 shows how the segmental order parameters vary for three different levels of hydration, and the variation of the 1H–13C dipolar splittings with nw is summarized in Fig. 3. A comparison of the order parameters in Fig. 2 with those obtained previously by using specifically deuterated DMPC (compiled in ref. 2b) indicates satisfactory agreement between 1H–13C APM-CP and 2H NMR results. The hydration properties of lipid headgroups have previously been characterized by 2H NMR using various 2H-labelled molecules.11 To the best of our knowledge, however, the result in Fig. 2 represents the first report of the ordering profile across the entire lipid versusnw. A complete conformational analysis employing statistical-mechanical methods12 will be presented elsewhere. However, some immediate remarks will be made. First, the acyl chains become more ordered upon dehydration. This is consistent with an increase of the average bilayer thickness when the water content is decreased.13 Second, the heteronuclear dipolar splittings produced by the α and β segments exhibit a counter-directional change with the hydration level. Similar observations made in 2H NMR investigations of phospholipids were explained by assuming that the headgroup moves closer to the membrane interior upon dehydration.11 Third, at a hydration level around 25–30 water molecules per lipid, the 1H–13C splittings reach a limiting plateau indicating full hydration of the DMPC bilayer.
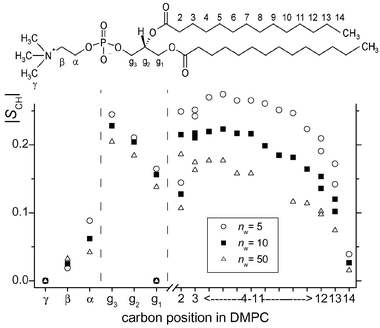 | ||
| Fig. 2 Local order parameters for the various segments in DMPC plotted for three different levels of hydration at 41 °C. | ||
 | ||
| Fig. 3 1H–13C dipolar splittings as a function of the hydration level in DMPC at 41 °C. For clarity, only 5 out of 18 available chain splittings are shown (more data are available in the electronic supplementary information).† The solid lines are drawn to guide the eye. | ||
In solid-state NMR studies of membrane-bound peptides and proteins, it is tempting to use strongly dehydrated lipid bilayers since this approach improves the mechanical stability of the sample, and reduces problems related to rf heating and poor signal intensity.6 Our study suggests that this tactics should be used with caution. Lowering the water content below nw ≈ 20 changes the structure of the lipid molecules, which may alter the membrane functioning.
To summarize, we have introduced a new SLF technique for precise measurements of 1H–13C dipolar couplings in anisotropic fluids under MAS. The pulse sequence can also be employed as a building block in advanced multi-dimensional experiments. The method has been used to investigate the orientational ordering profile of DMPC in the liquid crystalline phase as a function of the hydration level. The DMPC/water system is admittedly a simple model of a true cell membrane. The results presented in this communication show, nevertheless, that a wealth of high-quality molecular information can be extracted from unoriented and unlabelled lipid samples using APM-CP. Due to its robustness and high scaling factor, we believe that the novel recoupling scheme will become a widespread approach for characterization of soft materials such as membranes and liquid crystals.
Acknowledgements
This work was supported by the Swedish Research Council, the Magn. Bergvall Foundation, the Carl Trygger Foundation, and the Lars Hierta Foundation.Notes and references
- (a) J. Seelig, Q. Rev. Biophys., 1977, 10, 353 CrossRef CAS; (b) J. H. Davis, Biochim. Biophys. Acta, 1983, 737, 117 CAS.
- (a) M. Hong, K. Schmidt-Rohr and A. Pines, J. Am. Chem. Soc., 1995, 117, 3310 CrossRef CAS; (b) J. D. Gross, D. E. Warschawski and R. G. Griffin, J. Am. Chem. Soc., 1997, 119, 796 CrossRef CAS; (c) D. A. Middleton, E. Hughes and J. Madine, J. Am. Chem. Soc., 2004, 126, 9478 CrossRef CAS.
- (a) J. Forbes, J. Bowers, X. Shan, L. Moran, E. Oldfield and M. A. Moscarello, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 3821 RSC; (b) S. V. Dvinskikh, V. Castro and D. Sandström, Magn. Reson. Chem., 2004, 42, 875 CrossRef CAS.
- M. G. Munowitz, R. G. Griffin, G. Bodenhausen and T. H. Huang, J. Am. Chem. Soc., 1981, 103, 2529 CrossRef CAS.
- T. A. Cross and J. R. Quine, Concepts Magn. Reson., 2000, 12, 55 CrossRef CAS.
- F. M. Marassi and K. J. Crowell, J. Magn. Reson., 2003, 161, 64 CrossRef CAS.
- M. H. Levitt, in Encyclopedia of Nuclear Magnetic Resonance, ed. D. M. Grant and R. K. Harris, Wiley, Chichester, 2002, p. 165 Search PubMed.
- (a) S. V. Dvinskikh, H. Zimmermann, A. Maliniak and D. Sandström, J. Magn. Reson., 2004, 168, 194 CrossRef CAS; (b) S. V. Dvinskikh, H. Zimmermann, A. Maliniak and D. Sandström, J. Magn. Reson., 2003, 164, 165 CrossRef CAS; (c) S. V. Dvinskikh, H. Zimmermann, A. Maliniak and D. Sandström, J. Chem. Phys., 2005, 122, 044512 CrossRef; (d) S. V. Dvinskikh, V. Castro and D. Sandström, Phys. Chem. Chem. Phys., 2005, 7, 607 RSC; (e) S. V. Dvinskikh and D. Sandström, J. Magn. Reson., 2005, 175, 163 CrossRef CAS.
- (a) T. M. Barbara and E. H. Williams, J. Magn. Reson., 1992, 99, 439 CAS; (b) B. Q. Sun, P. R. Costa and R. G. Griffin, J. Magn. Reson., Ser. A, 1995, 112, 191 CrossRef CAS; (c) S. Hediger, B. H. Meier and R. R. Ernst, J. Chem. Phys., 1995, 102, 4000 CrossRef; (d) M. Bjerring and N. C. Nielsen, Chem. Phys. Lett., 2003, 382, 671 CrossRef CAS.
- N. C. Nielsen, H. Bildsoe, H. J. Jakobsen and M. H. Levitt, J. Chem. Phys., 1994, 101, 1805 CrossRef CAS.
- (a) B. Bechinger and J. Seelig, Chem. Phys. Lipids, 1991, 58, 1 CrossRef CAS; (b) A. S. Ulrich and A. Watts, Biophys. Chem., 1994, 49, 39 CrossRef CAS.
- B. Stevensson, D. Sandström and A. Maliniak, J. Chem. Phys., 2003, 119, 2738 CrossRef CAS.
- M. J. Janiak, D. M. Small and G. G. Shipley, J. Biol. Chem., 1979, 254, 6068 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Theoretical description of APM-CP, numerical line shape simulations, and additional experimental dipolar splittings. See http://dx.doi.org/10.1039/b508190d |
| This journal is © the Owner Societies 2005 |
