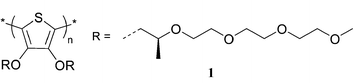
Non-ionic polythiophenes: a non-aggregating folded structure in water†
James R.
Matthews
,
Francesca
Goldoni
,
Albertus P. H. J.
Schenning
* and
E. W.
Meijer
*
Laboratory of Macromolecular and Organic Chemistry, Eindhoven University of Technology, PO Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: a.p.h.j.schenning@tue.nl; e.w.meijer@tue.nl
First published on 10th October 2005
Abstract
The non-aggregating nature of a water-soluble π-conjugated polythiophene has been characterised by concentration independent thermal denaturing.
π-Conjugated polymers are highly interesting materials that have potential applications as sensors1 for biological,2,3 organic4,5 and inorganic6–8 materials. Sensing relies on the photophysical properties of π-conjugated polymers changing in a reliable and reproducible way on exposure to analytes. However, π-conjugated polymers often form aggregates in an ill-defined way causing irreproducible sensing results. The use of non-aggregating isolated chain polymers having a specific conformation therefore has appeal. It is of the utmost importance to be able to distinguish between an aggregated and a non-aggregated folded species. This is a non-trivial issue. While the folding structure of oligomers may often be determined by NMR9 and X-ray crystallography, polymers present significant challenges to both these techniques. Polymers often show very broad NMR peaks and form amorphous films rather than crystallise.
Water-soluble π-conjugated polymers are of particular interest due to the ubiquity of water in biological contexts. In addition, these polymers should be non-ionic to maximise the chances of compatibility and minimise interference in sensing through non-specific ionic interactions. Non-ionic water soluble π-conjugated polymers have been synthesised and investigated.1,2,10 However, there are only a few claims in which the polymers are present as a single species.10 Herein we present the synthesis of a water soluble π-conjugated polythiophene having oligoethylene glycol side-chains (1) and its single chain conformation in aqueous solution, by investigating the concentration dependence of the thermal denaturation.
Polythiophene 1 was synthesised by oxidative coupling with iron(III) chloride followed by purification by size exclusion chromatography. The synthesis of the monomer is based on a transetherification with 3,4-dimethoxythiophene and a chirally substituted tetra-ethylene glycol.‡ The resulting purple polymer has been characterised, including by GPC and NMR.‡
The optical properties of the polymer have been investigated in THF and water. Upon dissolution in THF red solutions are produced, which exhibit spectra (λmax = 510 nm) similar to other polythiophenes having random coil conformations.11,12Fig. 1 shows the effect of varying the solvent from THF to pure water for solutions of polythiophene 1. As much as 96% water may be present without affecting the random coil like nature of the spectrum. With less than 4% THF in the now mostly aqueous solution the spectrum starts to shift. When dissolved in pure water the solutions are purple at room temperature having absorption maxima at 508 nm, 550 nm and 602 nm, clearly indicating a non-random coil conformation. Fig. 2 shows the UV–visible and fluorescence spectra of polythiophene 1 in both pure THF and pure water (λex = 502 nm). Not only does the aqueous UV–visible spectrum show additional red-shifted bands but the aqueous fluorescence (λmax,em = 612 nm, 662 nm) is also red-shifted in relation to that of the random coil conformation of the THF dissolved polymer (λmax,em = 590 nm). Upon heating an aqueous solution of polythiophene 1 the UV–visible absorption and fluorescence emission spectra change to ones similar to that of a THF solution (Figs. 3 and 4). The interpretation of spectroscopic data for such materials is usually ascribed to either aggregation13–15 or to non-aggregation processes involving the adoption of different conformers in solution.11,16,17 Considerable debate exists on this subject because foldamers exhibit many of the photophysical properties of aggregates.
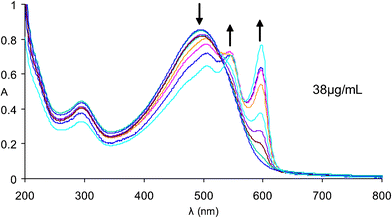 | ||
| Fig. 1 UV–visible spectra of polythiophene 1 in THF/water mixtures, ranging from 96%–100% water. | ||
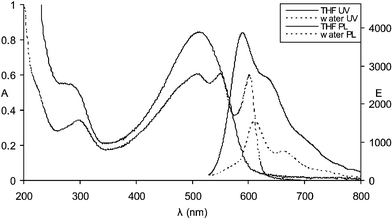 | ||
| Fig. 2 UV–visible and fluorescence spectra of polythiophene 1 in THF and in water (λex = 502 nm). | ||
 | ||
| Fig. 3 UV–visible spectra of polythiophene 1 in water from 10 °C to 60 °C. | ||
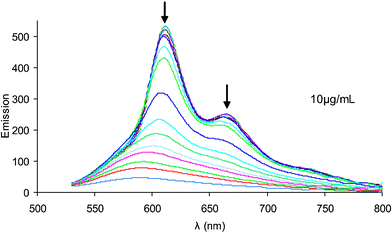 | ||
| Fig. 4 Fluorescence spectra of polythiophene 1 in water from 10 °C to 60 °C (λex = 502 nm). | ||
To investigate the issue of aggregation vs. conformation in detail concentration dependent UV–visible measurements were carried out. The intensity of the UV–visible absorption spectrum of polythiophene 1 changes linearly with concentration (Fig. 5). This is consistent with a non-aggregated behaviour. The linear change in absorption with concentration suggests that this is not an aggregate species in water. If, however, aggregation is very strong then dilution may not break up the aggregates to a significant enough extent. Additional proof is therefore required to rule out aggregation.
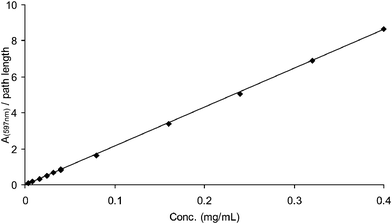 | ||
| Fig. 5 Linear change in the peak absorbance of polythiophene 1 in water. | ||
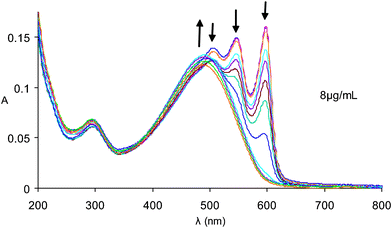
For an aggregated system the temperature at which the denaturation transition occurs should be dependent on the concentration of the solution. This has been shown to be the case for aggregated oligomeric systems,18,19 where increasing the concentration raises the temperature of thermal deaggregation. In contrast, the thermal unfolding of a single chain species should be independent of concentration. The UV–visible absorption spectrum of an aqueous solution of polythiophene 1 was monitored over a range of temperatures from 10 °C to 60 °C at a variety of different concentrations. Fig. 6 shows one of the peak absorbances (597 nm) over the temperature range observed, scaled by solution concentration, for four different concentrations.20 It can clearly be seen from the close congruence of the lines that the thermal transition is concentration independent, pointing to foldameric properties.21
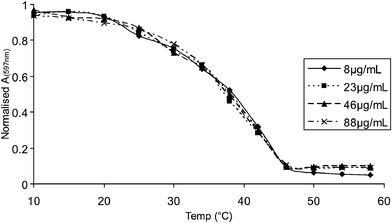 | ||
| Fig. 6 Concentration independence of the thermal unfolding transition of polythiophene 1 in water. | ||
Fig. 7 shows the concentration independence of the fluorescence of polythiophene 1 through the thermal transition between folded and unfolded states in water, revealing similar behaviour to that from the UV–visible spectra. However, the exact shape of the relative spectral intensities throughout the transition is slightly different from UV–visible spectra to fluorescence spectra.22 It is generally observed that as a solution of an aggregating polymer is cooled its fluorescence goes down due to quenching through inter-chain interactions. However, in the case of polythiophene 1 quite the reverse is seen. Fig. 7 shows a dramatic increase in fluorescence on cooling, revealing that this transition is more likely to be related to folding than to aggregation.
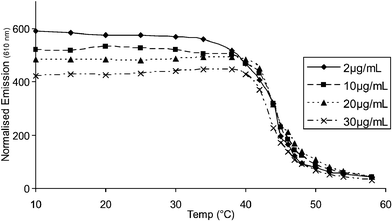 | ||
| Fig. 7 Fluorescence thermal unfolding transitions of polythiophene 1 in water (λex=502 nm). | ||
Aqueous GPC measurements have been made on polythiophene 1 with injection concentrations in the range 0.064–8 mg/mL.‡ In all cases the elution time was identical, indicating that no aggregation exists in aqueous solution.
Since polythiophene 1 has been shown here not to aggregate in aqueous solution one must consider what conformational structure it does adopt. Circular dichroism experiments showed no Cotton effect for polythiophene 1 in water, in contrast to earlier findings for aggregated polythiophenes.15 The lack of a Cotton effect indicates that the chirality in the side-chains is not expressed in the main-chain and that in the case of a folded structure equal amounts of P and M helices exist. This is evidence for a structure that does not bring the side-chains into close proximity. The broad nature of the NMR signals for polythiophene 1 in D2O makes NOE experiments to determine conformation in solution impossible. Dilution of the solutons in D2O did not lead to significant changes in the shape or positions of the peaks. Interestingly, the spectrum is similar to that in deuterated chloroform, suggesting that the side-chains are not packed in water. Both Moore23 and Hecht10,24 have made oligoethylene glycol appended foldamers in aqueous solution and shown them to have a helical conformation. Calculations have shown that the helical form of an all cis polythiophene is a stable conformation.25 Based on this we believe that polythiophene 1 adopts a helical conformation in water.
A new polythiophene has been synthesised that shows a non-aggregating folded structure in water. This behaviour was determined by new techniques for distinguishing between foldameric and aggregated polymeric systems.26 The new π-conjugated polythiophene presented here provides a basis for the development of new and interesting materials with controlled electronic properties through control of their secondary structure, which will have applications in the field of organic electronics as well as biosensing.
Koen Pieterse is acknowledged for generating the artwork that is on the cover of this issue.
Notes and references
- D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537–2574 CrossRef CAS.
- M. D. Disney, J. Zheng, T. M. Swager and P. H. Seeberger, J. Am. Chem. Soc., 2004, 126, 13343–13346 CrossRef CAS.
- C. H. Fan, K. W. Plaxco and A. J. Heeger, J. Am. Chem. Soc., 2002, 124, 5642–5643 CrossRef CAS.
- J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864–11873 CrossRef CAS.
- Q. Zhou and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 7017–7018 CrossRef.
- P. Bauerle and S. Scheib, Acta Polym., 1995, 46, 124–129 CrossRef.
- B. Wang and M. R. Wasielewski, J. Am. Chem. Soc., 1997, 119, 12–21 CrossRef.
- G. Rimmel and P. Bauerle, Synth. Met., 1999, 102, 1323–1324 CrossRef CAS.
- J. C. Nelson, J. G. Saven, J. S. Moore and P. G. Wolynes, Science, 1997, 277, 1793–1796 CrossRef CAS.
- A. Khan, S. Müller and S. Hecht, Chem. Commun., 2005, 584–586 RSC.
- K. Faid, M. Frechette, M. Ranger, L. Mazerolle, I. Levesque, M. Leclerc, T. A. Chen and R. D. Rieke, Chem. Mater., 1995, 7, 1390–1396 CrossRef.
- G. W. Heffner and D. S. Pearson, Macromolecules, 1991, 24, 6295–6299 CrossRef CAS.
- S. Yue, G. C. Berry and R. D. McCullough, Macromolecules, 1996, 29, 933–939 CrossRef CAS.
- R. D. McCullough, P. C. Ewbank and R. S. Loewe, J. Am. Chem. Soc., 1997, 119, 633–634 CrossRef CAS.
- B. M. W. Langeveld-Voss, R. A. J. Janssen and E. W. Meijer, J. Mol. Struct., 2000, 521, 285–301 CrossRef CAS.
- G. Daoust and M. Leclerc, Macromolecules, 1991, 24, 455–459 CrossRef CAS.
- N. Kiriy, E. Jahne, H. J. Adler, M. Schneider, A. Kiriy, G. Gorodyska, S. Minko, D. Jehnichen, P. Simon, A. A. Fokin and M. Stamm, Nano Lett., 2003, 3, 707–712 CrossRef CAS.
- J. J. Apperloo, R. A. J. Janssen, P. R. L. Malenfant and J. M. J. Frechet, Macromolecules, 2000, 33, 7038–7043 CrossRef CAS.
- P. Jonkheijm, F. J. M. Hoeben, R. Kleppinger, J. van Herrikhuyzen, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2003, 125, 15941–15949 CrossRef CAS.
- The non S-like nature of the curve suggests that there is some degree of cooperativity in the folding process.
- When applied to systems known to be aggregated, such as solutions of poly-3-hexylthiophene in chloroform/n-hexane, the thermal transition can be seen to change with different concentrations.
- The denaturation of polythiophenes in methanol has previously been investigated by comparing thermal transitions between UV–visible and fluorescence spectra, revealing similar results. I. Levesque and M. Leclerc, Chem. Mater., 1996, 8, 2843–2849 Search PubMed.
- M. T. Stone and J. S. Moore, Org. Lett., 2004, 6, 469–472 CrossRef CAS.
- A. Khan and S. Hecht, Synth. Met., 2004, 147, 37–42 CrossRef CAS.
- C. X. Cui and M. Kertesz, Phys. Rev. B, 1989, 40, 9661–9670 CrossRef CAS.
- Experiments covering extension of the concentration dependent thermal denaturation technique to other polymers have been carried out but the material has yet to be published.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental details, synthetic procedures and characterisation of products and intermediates. See DOI: 10.1039/b512119a |
| ‡ Synthetic procedures and experimental details are included in the ESI. |
| This journal is © The Royal Society of Chemistry 2005 |