A quinone-functionalized electrode in conjunction with hydrophobic magnetic nanoparticles acts as a “Write–Read–Erase” information storage system
Eugenii
Katz
and
Itamar
Willner
*
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem, 91904, Israel. E-mail: willnea@vms.huji.ac.il; Fax: 972-2-6527715; Tel: 972-2-6585272
First published on 18th October 2005
Abstract
Integration of hydrophobic magnetic nanoparticles with a quinone-functionalized Au electrode in a water–toluene two-phase assembly yields a “Write–Read–Erase” information processing system.
Continuous research efforts are directed towards the development of chemical ensembles exhibiting “Write–Read–Erase” functions for information storage and processing.1,2 Different stimuli including light signals,3 electrical4 or chemical5 inputs were employed as signals for the activation of “Read–Write–Erase” systems. The integration of molecular functions with surfaces, and specifically electrodes, allowed the coupling of different signals to design information storage and readout systems. For example, the photochemical isomerization of an azobenzene monolayer was used to “write” information while the redox activity of one photoisomer state was used to “read” and “erase” the recorded information.6 Similarly, the photochemical isomerization of a phenoxynaphthacene quinone monolayer associated with an electrode was used to record photonic signals and subsequently to read-out the registered information by electrochemical means, and then to erase the information by a photochemical transformation.7 Also, electrochemically induced reduction of WO3 was used to record information in a solid support, and to read-out the information by optical means.8
Magnetic nanoparticles (NPs) capped with a hydrophobic layer were recently employed to control the hydrophilic/hydrophobic properties of surfaces,9 and to switch the redox features at electrode supports.10 Here we wish to report on an electrochemically activated system, which is gated by hydrophobic magnetic NPs, that reveals “Write–Read–Erase” functions.
Scheme 1 depicts the operating principles of the information processing system. The system consists of a quinone-functionalized electrode array in conjunction with a two-phase aqueous/toluene system. Au-coated (50 nm) glass slides (Analytical μ-Systems, Germany) were used as conductive supports and placed at the bottom of the electrochemical cell. The Au layer was scratched prior to the surface modification to divide the conductive support into two individually wired Au electrodes (ca. 0.15 cm2 each electrode). The Au electrodes were modified by an aminonaphthoquinone derivative (1) covalently bound to a cystamine monolayer self-assembled on Au electrodes.11 The aqueous layer, 2 ml, is composed of a phosphate buffer (0.1 M, pH = 7.0) and it operates as the electrolyte solution. Magnetite NPs, Fe3O4, average diameter ca. 5 nm, were capped with a hydrophobic layer of undecanoic acid (saturated magnetization 36.4 emu g−1), and the NPs were solubilized in toluene (0.5 mL, 1 mg mL−1), yielding an upper organic solution layer immiscible with the aqueous electrolyte solution (ca. 1.5 cm thickness of the aqueous layer and ca. 4 mm thickness of the toluene phase).9,10 The hydrophobic magnetic NPs were attracted to the electrode surface from the upper organic layer by positioning a 12-mm diameter NdFeB/Zn-coated magnet with the remanent magnetization of 10.8 kG below the bottom electrode, and were removed from the electrode surface and re-transported to the organic phase by positioning the external magnet on the top of the electrochemical cell. The hydrophobic NPs magnetically transported to the electrode surface carried co-adsorbed toluene solvent (ca. 410 toluene molecules per NP) and generated a thin non-aqueous film (ca. 8.5 µm) on the electrode surface.10 When the magnetic NPs with the associated toluene molecules were re-transported to the upper toluene phase, the quinone-functionalized Au electrode surface was again exposed to the aqueous electrolyte solution. The significant difference in the electrochemical behavior of the quinone monolayer in the aqueous and non-aqueous environments,12 and the possibility of exchanging reversibly the aqueous and non-aqueous environments at the electrode surface by the attraction/retraction of the magnetic NPs, were used to develop the “Write–Read–Erase” system.
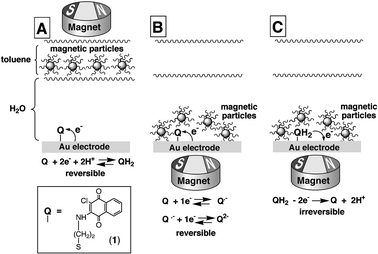 | ||
| Scheme 1 “Write–Read–Erase” electrochemical system based on the quinone-monolayer-modified Au electrode with the interfacial properties controlled by the hydrophobic magnetic NPs. (A) “Write” process in the aqueous environment (magnetic NPs are retracted from the electrode). (B) “Read” process in the non-aqueous environment (magnetic NPs are attracted to the electrode). (C) “Erase” process in the non-aqueous environment (magnetic NPs are attracted to the electrode). | ||
The process is started when the hydrophobic magnetic NPs are confined to the organic phase and the electrode interface is exposed to the aqueous electrolyte solution, Scheme 1(A). Fig. 1 (curve a) shows the cyclic voltammogram of the 1-functionalized electrode (the cyclic voltammograms are identical for the right and left Au electrodes). The characteristic reversible 2e−/2H+-redox wave of the quinone monolayer, E° = −0.41 V (all potentials are referred to SCE), exposed to the aqueous electrolyte is observed. The surface coverage of the quinone units associated with each electrode corresponds to 2.2 × 10−11 mole cm−2. The information was “written” in the system by applying different potentials: E1 = −0.2 V corresponding to the oxidized state of the quinone units (Q) and E2 = −0.6 V when the quinone is reduced to the hydroquinone form (QH2). These two potentials were applied on the two quinone-modified Au electrodes exposed to the aqueous electrolyte solution resulting in the oxidized (Q) and reduced (QH2) forms on the left and right electrodes, respectively. The hydrophobic magnetic NPs were then attracted to the modified Au electrodes while applying the respective potentials, to perform the “read” process.
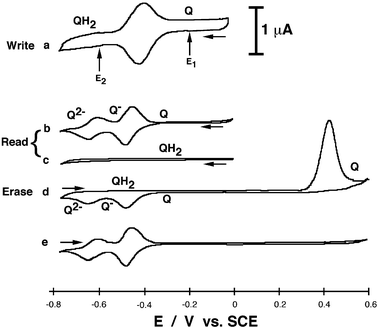 | ||
| Fig. 1 Cyclic voltammograms recorded in the presence of: a) Magnetic NPs retracted from the electrode and the applied potentials (E1 or E2) are used to “write” the information on the left and right electrodes, respectively. b) Magnetic NPs attracted to the electrodes: “Read” process on the right Au electrode. c) Magnetic NPs attracted to the electrodes: “Read” process on the left Au electrode. d) Magnetic NPs attracted to the electrodes: “Erase” process on the right Au electrode. e) Magnetic NPs attracted to the electrodes: Second scan after the “Erase” process on the right Au electrode. The data were obtained under Ar in a biphase system consisting of 0.1 M phosphate buffer, pH 7.0, and toluene with the magnetic NPs, 0.5 mL, 1 mg mL−1. Potential scan rate 100 mV s−1. Arrows show the direction of the potential sweep. | ||
The “read” process was performed in the non-aqueous environment provided by the hydrophobic magnetic NPs attracted to the electrodes, Scheme 1(B). Fig. 1 (curve b) shows the cyclic voltammogram recorded on the left electrode, that included the magnetic NPs on the oxidized quinone surface (Q). The cyclic voltammogram exhibits two consecutive redox waves corresponding to the 1e−-reduction/oxidation steps each, E°1 = −0.465 V and E°2 = −0.630 V, characteristic to the immobilized quinone units in the non-aqueous environment, Scheme 1(B). Fig. 1 (curve c) shows the cyclic voltammogram of the right electrode that includes the hydrophobic magnetic NPs on the reduced hydroquinone interface (QH2). It reveals a low capacitance and lacks any redox waves. The modified electrode is in the mute, electrochemically non-active state within the applied potential range. This originates from the fact that the reduced hydroquinone state, that was generated in the aqueous environment, cannot be further reduced, and its oxidation in the non-aqueous environment requires a large overpotential. Thus, the information “written” on the left and right modified electrodes in the aqueous environment can be “read” out by electrochemical means in the non-aqueous environment in the presence of the attracted magnetic NPs. It should be noted that the “read” process can be repeated by cyclic voltammetry within the defined potential range, without “erasing” the “written” information.
At this point, there are two possibilities to “erase” the “written” information. (i) The system can be returned to the aqueous environment by the retraction of the magnetic NPs, thus allowing the reversible 2e−/2H+-electrochemical process resulting in the easy release of H+ ions, that locked the reduced hydroquinone form in the non-aqueous environment in the electrochemically mute state. In this case both modified electrodes show again similar cyclic voltammograms, Fig. 1 (curve a), and the electrode array can be used again for the “write” process. (ii) A positive potential-scan, up to +0.6 V, can be applied on the right electrode, which is electrochemically mute at negative potentials. The cyclic voltammogram, Fig. 1 (curve d), shows no peaks till the potential reaches the value of ca. +0.44 V, where irreversible oxidation of the hydroquinone occurs releasing the electrochemically active oxidized quinone form (Q), Scheme 1(C). Upon continuing the cyclic voltammogram in the negative direction, two consecutive redox waves corresponding to the 1e−-reduction steps of the immobilized quinone (1) are observed. This is consistent with the fact that the electrochemically mute hydroquinone form was electrochemically converted to the redox-active quinone upon its irreversible oxidation at the positive potential. The second potential-scan in the positive direction shows already at negative potentials the redox process characteristic to the quinone units in the non-aqueous environment, but the irreversible anodic peak at E = +0.44 V is not observed in the second cycle, Fig. 1 (curve e). This is consistent with the fact that the hydroquinone form, that is oxidizable at this potential, is not generated in the presence of the hydrophobic magnetic NPs. The irreversible electrochemical oxidation of the hydroquinone form, which is mute at the negative potentials, yields the electrochemically active quinone that can be reduced. Although this results in the “erasing” of the “written” information, a new “write” process is impossible till the magnetic NPs are removed from the electrode surface and the electrode is exposed to the aqueous environment. The system demonstrates remarkable stability and reproducibility, allowing many cycles of the “write–read–erase” process (e.g. less than 5% decrease of the peaks in the cyclic voltammograms was observed after 50 cycles).
The present study has allowed the coupling of a quinone-functionalized electrode with hydrophobic magnetic NPs in an aqueous/toluene two-phase system to design a novel concept for a “Write–Read–Erase” system. The “Write” function was achieved by two different potential inputs (E1 or E2), while the physical attraction of the hydrophobic magnetic NPs to the electrode enabled the electrochemical readout of the recorded information. The “Erase” process was accomplished electrochemically either in the presence of the magnetic NPs on the electrode surface, or upon their removal from the surface. One may envisage the future development of electrochemical information storage and processing systems based on this concept, and their application as multi-electrode micro-chip devices.
This research is supported by the Israel–China Binational Program, The Israel Ministry of Science.
Notes and references
- (a) R. Ballardini, V. Balzani, A. Credi, M. T. Gandolfi and A. Venturi, Acc. Chem. Res., 2001, 34, 445–455 CrossRef CAS; (b) A. P. de Silva and N. D. McClenghan, Chem. Eur. J., 2004, 10, 574–586 CrossRef.
- (a) I. Willner, Acc. Chem. Res., 1997, 30, 347–356 CrossRef CAS; (b) J. Andreasson, G. Kodis, Y. Terazoni, P. A. Liddell, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2004, 126, 15926–15927 CrossRef CAS.
- (a) S. L. Gilat, S. H. Kawai and J.-M. Lehn, Chem. Eur. J., 1995, 1, 275–284 CrossRef CAS; (b) P. A. Liddell, G. Kodis, J. Andreasson, L. de La Ganza, S. Bandyopadhyay, R. H. Mitchell, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2004, 126, 4803–4811 CrossRef.
- (a) M. Asakawa, P. R. Ashton, V. Balzani, A. Credi, G. Mattersteig, O. A. Matthews, M. Montalti, N. Spencer, J. F. Stoddart and M. Venturi, Chem. Eur. J., 1997, 3, 1992–1996 CrossRef CAS; (b) E. Katz, O. Lioubashevski and I. Willner, J. Am. Chem. Soc., 2004, 126, 15520–15532 CAS.
- A. Credi, V. Balzani, S. J. Langford and J. F. Stoddart, J. Am. Chem. Soc., 1997, 119, 2679–2681 CrossRef CAS.
- Z. F. Liu, K. Hashimoto and A. Fujishima, Nature, 1990, 347, 658–660 CrossRef CAS.
- A. Doron, M. Portnoy, M. Lion-Dagan, E. Katz and I. Willner, J. Am. Chem. Soc., 1996, 118, 8937–8944 CrossRef CAS.
- I. Turyan, U. O. Krasovec, B. Orel, T. Saraidorov, R. Reisfeld and D. Mandler, Adv. Mater., 2000, 12, 330–333 CrossRef CAS.
- E. Katz, L. Sheeney-Haj-Ichia, B. Basnar, I. Felner and I. Willner, Langmuir, 2004, 20, 9714–9719 CrossRef CAS.
- E. Katz, R. Baron and I. Willner, J. Am. Chem. Soc., 2005, 127, 4060–4070 CrossRef CAS.
- E. Katz and A. A. Solov'ev, J. Electroanal. Chem., 1990, 291, 171–186 CrossRef CAS.
- J. Q. Chambers, in: The Chemistry of the Quinonoid Compounds, S. Patai, (Ed.), Interscience, New York, 1974, p. 739 Search PubMed.
| This journal is © The Royal Society of Chemistry 2005 |
