Dynamic multivalent recognition of cyclodextrin vesicles†
Choon Woo
Lim
,
Bart Jan
Ravoo
* and
David N.
Reinhoudt
*
Laboratory of Supramolecular Chemistry and Technology, MESA+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands. E-mail: b.j.ravoo@utwente.nl; Fax: +31 53 489 4645; Tel: +31 53 489 2980
First published on 21st October 2005
Abstract
Cyclodextrin bilayer vesicles have dynamic membranes that recognize guest molecules through efficient multivalent host–guest interaction reminiscent of multivalent binding of a ligand with receptors in a biological membrane.
Receptor clustering is a powerful tool that cells and bacteria use to tune affinity and select competing ligands on their membrane surface.1 In chemical terms, this phenomenon is an example of dynamic molecular recognition through multivalent interaction.2 Here we describe an artificial membrane with embedded receptor molecules that recognizes and binds a suitable ligand via multivalent interaction with a small cluster of receptors. Previously, we have reported the preparation of amphiphilic cyclodextrins (CDs) and the corresponding CD bilayer vesicles, which have the ability to recognize and bind specific guests.3 In this communication we report that a vesicle membrane composed of β-CD host molecules 1b has specific, multivalent interactions with a dye-labeled, divalent guest 3. Fluorescence resonance energy transfer (FRET)4 was used to monitor the complexation of fluorescent guest molecules to the CD vesicles. In vesicles composed of a minority of β-CD host 1b in a majority of “inert” α-CD 1a, clustering of β-CD host 1b leads to efficient multivalent interaction. We use a quantitative model to interpret the clustering of receptors as an increased effective concentration of receptor molecules at the membrane surface.
Fig. 1 shows a schematic illustration of a bilayer vesicle composed of amphiphilic CDs and the structure of amphiphilic CDs 1a and 1b. Fig. 1 also shows N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled cholesterol (NBD-Chol) 2, which is well known as a hydrophobic membrane probe,5 and N-(lissamine-rhodamine B)-labeled divalent adamantyl guest (LRB-Ad2) 3.6 NBD and LRB dyes are well known as a FRET donor–acceptor pair in studies of biological and model membranes.4
 | ||
| Fig. 1 Bilayer vesicle composed of amphiphilic CDs and molecular structure of amphiphilic CDs 1a and 1b, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled cholesterol (NBD-Chol) 2 and N-(lissamine-rhodamine B)-labeled divalent adamantane guest (LRB-Ad2) 3. | ||
Using conventional extrusion methods, we prepared unilamellar CD vesicles (diameter ca. 160 nm) containing 1 mol% NBD-Chol 2. The formation of bilayer vesicles from amphiphilic CDs 1a and 1b in water was observed by transmission electron microscopy and dynamic light scattering consistent with previous reports.3
Fig. 2 shows the steady-state fluorescence changes upon adding guest LRB-Ad23 (dissolved in 5 mM phosphate buffer, pH 7.5, 0.5% DMSO) to the host vesicle solution (10 µM CD 1b in 5 mM phosphate buffer and pH 7.5) containing 1 mol% NBD-Chol 2. The fluorescence intensity of donor 2 (λex = 450 nm, λem = 530 nm) decreased while the intensity of acceptor 3 (λem = 585 nm) increased upon addition of 3. The emission of 2 was nearly completely quenched upon addition of < 1 µM guest 3, indicating that efficient FRET occurred as a result of the interaction between guest 3 and β-CD host 1b, which brings acceptor 3 in close proximity to donor 2. The Förster distance (R0) for NBD and LRB ranges from 50 to 70 Å.4 According to the dimensions of the CD bilayer provided by X-ray diffraction data and Langmuir Blodgett isotherms,3 acceptor guest molecule 3 and donor membrane probe 2 will approach within the Förster distance upon complexation of 3 at the surface of vesicles of CD host 1b containing 2. Since the concentration of acceptor 3 was very low ([3]max = 0.9 µM), inner filter effects and collisional quenching will be negligible. Note also that in all experiments the concentration of guest 3 was much lower than host 1b.
![Fluorescence emission spectra (λex
= 450 nm) of vesicles of β-CD 1b
(10 µM) containing 1 mol% NBD-Chol 2
(0.1 µM) upon adding divalent guest LRB-Ad23. [3]
= 0–0.9 µM. All measurements were carried out in 5 mM phosphate buffer at pH = 7.5 and T
= 25 °C.](/image/article/2005/CC/b510540d/b510540d-f2.gif) | ||
| Fig. 2 Fluorescence emission spectra (λex = 450 nm) of vesicles of β-CD 1b (10 µM) containing 1 mol% NBD-Chol 2 (0.1 µM) upon adding divalent guest LRB-Ad23. [3] = 0–0.9 µM. All measurements were carried out in 5 mM phosphate buffer at pH = 7.5 and T = 25 °C. | ||
To visualize the interaction between guest 3 and vesicles of host 1b, confocal microscopy images of CD vesicles (10 µM) containing 1 mol% NBD-Chol 2 (0.1 µM) in the absence and presence of guest 3 were collected on a Carl Zeiss LSM510 microscope (Fig. 3). Fluorescence images were obtained by recording the red emission (above 560 nm) and the green emission (between 500 nm and 550 nm), while exciting at 458 nm. Hydrophobic probe 2 is exclusively localized in the CD vesicles (see ESI for additional evidence). In the absence of guest 3, the vesicles are observed in both emission channels as a result of the broad emission spectrum of NBD-Chol 2. However, when 1 µM guest 3 was added to the NBD-Chol 2 containing vesicle solution (10 µM), fluorescent vesicles were observed only in the red emission channel, indicating that the green fluorescence of 2 embedded in the vesicles was quenched via a FRET mechanism induced by binding guest 3 to β-CD 1b on the surface of the vesicles. The intense red fluorescence observed results from acceptor 3.
 | ||
| Fig. 3 Confocal microscopy images of vesicles of β-CD 1b. Scale: 207 × 207 µm for each window. (a) Vesicles of β-CD 1b containing NBD-Chol 2. (b) LRB-Ad23 bound to vesicles of β-CD 1b containing NBD-Chol 2. Top: λem = 500–550 nm. Bottom: λem > 560 nm. See text for details. | ||
In a negative control experiment, LRB without a divalent adamantane anchor was added to vesicles containing donor 2. Emission spectra showed that there was no significant FRET between 2 in the β-CD vesicle and LRB in bulk solution (see ESI). LRB-Ad23 was also added to vesicles of α-CD 1a and to non-CD vesicles3 composed of n-dodecyl triethyleneglycol (C12EO3, 90%) and n-tetradecyl triethyleneglycol (C14EO3, 10%), both containing NBD-Chol 2 (0.1 µM). Neither the vesicles of α-CD 1a (which is too small to fit adamantane) nor the non-CD vesicles (which do not contain host molecules) show any change of fluorescence of NBD-Chol 2 upon addition of divalent guest 3. The Stern–Volmer plot (Fig. 4) demonstrates that the recognition of guest 3 by the vesicles is specifically mediated by the host–guest complexation of adamantane guest 3 and CD host 1b at the vesicle surface.
![NBD-Chol 2 fluorescence intensity (F0/F)
versus guest concentration: (∇) Vesicles of β-CD 1b and LRB-Ad23. Negative controls: (▼) Vesicles of β-CD 1b and LRB; (●) Vesicles of C12EO3/C14EO3 and LRB-Ad23; (○) Vesicles of α-CD 1a and LRB-Ad23. [NBD-Chol 2]
= 0.1 µM and [1a]
=
[1b]
= 10 µM. [C12EO3/C14EO3]
= 100 µM. All measurements were carried out in 5 mM phosphate buffer at pH = 7.5 and T
= 25 °C.](/image/article/2005/CC/b510540d/b510540d-f4.gif) | ||
| Fig. 4 NBD-Chol 2 fluorescence intensity (F0/F) versus guest concentration: (∇) Vesicles of β-CD 1b and LRB-Ad23. Negative controls: (▼) Vesicles of β-CD 1b and LRB; (●) Vesicles of C12EO3/C14EO3 and LRB-Ad23; (○) Vesicles of α-CD 1a and LRB-Ad23. [NBD-Chol 2] = 0.1 µM and [1a] = [1b] = 10 µM. [C12EO3/C14EO3] = 100 µM. All measurements were carried out in 5 mM phosphate buffer at pH = 7.5 and T = 25 °C. | ||
The slope of the Stern–Volmer plot can be employed to estimate the apparent association constant Ka = 1.5 × 107 M−1 between host 1b and divalent guest 3.‡ The magnitude of this association constant is diagnostic for a divalent interaction of one guest molecule with two host molecules. The efficient divalent interaction of guest 3 with host 1b is characterised by the equilibrium constant Ka2 = Ceff × Ka12 where Ka1 is the monovalent association constant and Ceff is the effective concentration of host molecules 1b at the surface of the CD vesicle.7 Ceff reflects the high number of β-CD hosts on the membrane surface accessible to the second adamantyl group, after the first adamantyl group of guest 3 binds to the vesicle. Ceff was calculated from a straightforward geometrical model taking into account the experimental molecular surface area of the CD host molecule 1b3 and the distance between the two adamantyl groups at guest 37 (see ESI). According to this model, Ceff = 0.17 M, which is consistent with the value of Ceff of CD host molecules at the surface of densely packed CD monolayers on gold and glass.6,7 Assuming that the divalent interaction Ka2 is the result of two equal and independent monovalent interactions Ka1, it follows that Ka1 = (Ka2/Ceff)0.5 = 9.4 × 103 M−1, which is consistent with the experimental value for the monovalent interaction of 1b with adamantane carboxylate (Ka1 = 7.0 × 103 M−1).3 We conclude that the recognition of guest 3 by host 1b is amplified by the formation of a divalent host–guest complex at the membrane surface.§
Furthermore, we measured the apparent association constant Ka of guest 3 with CD vesicles composed of mixtures of “good” host CD 1b and “inert” CD 1a (Table 1). CDs 1a and 1b differ in ring size only and can be readily mixed in all proportions. Since the alkyl chains of 1a and 1b are relatively short (C12), the bilayer membranes are in a liquid crystalline-like Lα phase with rapid lateral diffusion of the cyclodextrins on the vesicle surface. We therefore assume that the vesicle surface is a dynamic, homogeneous, two-dimensional solution with fractions of 1a and 1b directly proportional to their ratio in the mixture and a random distribution of 1a and 1b across the vesicle surface.
| Vesicle compositiona | 100 | 70 | 50 | 30 | 10 |
|---|---|---|---|---|---|
| a Percentage of β-CD 1b in mixed vesicles with α-CD 1a. b Effective concentration Ceff derived from Ka. Error ±10%. See text for details. c Calculated effective concentration Ceff for a statistical mixture of β-CD 1b and α-CD 1a. Error ±10%. See ESI for details. | |||||
| K a (M−1) | 1.5 × 107 | 1.2 × 107 | 1.2 × 107 | 1.1 × 107 | 3.5 × 106 |
| Ceff (M)b | 0.17 | 0.14 | 0.14 | 0.12 | 0.04 |
| Ceff (M)c | 0.17 | 0.11 | 0.08 | 0.04 | 0.002 |
Although Ka decreases with the mole percentage of 1b, Ka is still > 1 × 106 M−1 at a low percentage of 1b, indicative of divalent rather than monovalent interaction. Table 1 lists the value of Ceff calculated from Ka = Ka2 and Ka1 = 9.4 × 103 M−1 according to Ceff = Ka2/(Ka1)2. Finally, Table 1 lists the values of Ceff calculated from the experimental molecular surface area of CDs 1a and 1b3 and the distance between the two adamantane groups at guest 3 (see ESI). The values of Ceff as a function of the percentage of 1b in the CD vesicles are also shown in Fig. 5. It can be seen from Fig. 5 that Ceff as calculated from the apparent binding constant Ka deviates strongly and positively from Ceff as calculated for a statistical mixture of 1a and 1b. In other words, the effective concentration Ceff of host 1b in a mixture with inert 1a experienced by a suitable divalent guest molecule is much higher than expected for a random, statistical mixture of 1a and 1b. A plausible and appealing explanation for the high Ceff of 1b is that divalent guest 3 induces clustering of 1b in an excess of 1a.8 The small entropy cost of clustering will be easily offset by the large free energy gain of forming a divalent inclusion complex. However, we can not rule out (neither a priori nor by experiment) that some cluster formation occurs in the mixed CD vesicles even in the absence of divalent guest 3.
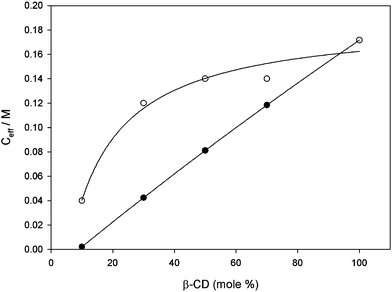 | ||
| Fig. 5 Experimental (○) and calculated (●) effective concentration (Ceff) of β-CD 1b experienced by guest 3versus percentage of β-CD 1b in vesicles composed of mixtures of CDs 1a and 1b. See text for details. | ||
In conclusion, we have demonstrated dynamic multivalent molecular recognition in an artificial membrane, reminiscent of the way that cells and bacteria select ligands on their membrane surface.
We thank Alart Mulder for the synthesis of 3 and Jurriaan Huskens for helpful discussions.
Notes and references
- R. N. Germain, Curr. Biol., 1997, 7, R640–R644 CrossRef CAS; D. Bray, M. D. Levin and C. J. Morton-Firth, Nature, 1998, 393, 85–88 CrossRef CAS; R. Willmann and C. Fuhrer, Cell. Mol. Life Sci., 2002, 59, 1296–1316 CrossRef CAS.
- M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754–2794 CrossRef; L. L. Kiessling, J. E. Gestwicki and L. E. Strong, Curr. Opin. Chem. Biol., 2000, 4, 696–703 CrossRef CAS.
- B. J. Ravoo and R. Darcy, Angew. Chem., Int. Ed., 2000, 39, 4324–4326 CrossRef CAS; B. J. Ravoo, J.-C. Jacquier and G. Wenz, Angew. Chem., Int. Ed., 2003, 42, 2066–2070 CrossRef CAS; P. Falvey, C. W. Lim, R. Darcy, T. Revermann, U. Karst, M. Giesbers, A. T. M. Marcelis, A. Lazar, A. W. Coleman, D. N. Reinhoudt and B. J. Ravoo, Chem.-Eur. J., 2005, 11, 1171–1180 CrossRef CAS.
- P. Wu and L. Brand, Anal. Biochem., 1994, 218, 1–13 CrossRef CAS; J. R. Lakowicz, Principles of Fluorescence Spectroscopy; Kluwer/Plenum Publishers: New York, USA, 1999 Search PubMed.
- R. Rukmini, S. S. Rawat, S. C. Biswas and A. Chattopadhyay, Biophys. J., 2001, 81, 2122–2134 CrossRef CAS.
- S. Onclin, A. Mulder, J. Huskens, B. J. Ravoo and D. N. Reinhoudt, Langmuir, 2004, 20, 5460–5466 CrossRef CAS; A. Mulder, S. Onclin, M. Péter, J. P. Hoogenboom, H. Beijleveld, J. ter Maat, M. F. García-Parajó, B. J. Ravoo, J. Huskens, N. F. van Hulst and D. N. Reinhoudt, Small, 2005, 1, 242–253 Search PubMed.
- A. Mulder, T. Auletta, A. Sartori, S. Del Ciotto, A. Casnati, R. Ungaro, J. Huskens and D. N. Reinhoudt, J. Am. Chem. Soc., 2004, 126, 6627–6636 CrossRef CAS; J. Huskens, A. Mulder, T. Auletta, C. A. Nijhuis, M. J. W. Ludden and D. N. Reinhoudt, J. Am. Chem. Soc., 2004, 126, 6784–6797 CrossRef CAS.
- It is well known that complexation of ions to lipids can induce clustering and phase separation in membranes composed of a mixture of lipids: H. J. Galla and E. Sackmann, J. Am. Chem. Soc., 1975, 97, 4114–4120 Search PubMed; D. M. Haverstick and M. Glazer, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 4475–4479 CrossRef CAS; K. M. Maloney, D. R. Schnek, D. Y. Sasaki and F. H. Arnold, Chem. Biol., 1996, 3, 185–192 CAS; V. Marchi-Artzner, M. J. Brienne, T. Gulik-Krzywicki, J. C. Dedieu and J. M. Lehn, Chem.-Eur. J., 2004, 10, 2342–2350 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: control experiments and calculation of the effective concentration Ceff of CD in mixed vesicles. See DOI: 10.1039/b510540d |
| ‡ The efficient divalent interaction of LRB-Ad23 with the CD vesicles is in marked contrast to inclusion interaction of polyanionic guest polymers with the CD vesicles.3 These guest polymers bind to the vesicles in a brush- or mushroom-type conformation with a Ka = 2 × 106 M−1 at most. It is likely that the oligo(ethylene glycol) residues protruding from the surface of the vesicles prevent optimal multivalent interaction with the guest polymers. This type of steric repulsion is well known for colloids and surfaces decorated with poly(ethylene glycol). Also there is a degree of electrostatic repulsion between the polyanion and the vesicles, which have a negative surface potential.3 |
| § The apparent association constant Ka is determined from the equation: F0/F = 1 + Ka[3], where F and F0 are the fluorescence intensity of donor NBD-Chol 2 in the presence and the absence of acceptor LRB-Ad23 and [3] is the concentration of 3. |
| This journal is © The Royal Society of Chemistry 2005 |
