Stereospecific change in conformation upon complexation of an exoditopic tetraamide host with a bis(ammonium) guest: chiral recognition and strong CD signaling†
Ryo
Katoono
,
Hidetoshi
Kawai
,
Kenshu
Fujiwara
and
Takanori
Suzuki
*
Division of Chemistry, Graduate School of Science, Hokkaido University, Sapporo 060-0810, Japan. E-mail: tak@sci.hokudai.ac.jp; Fax: +81 11 706-2714; Tel: +81 11 706-2714
First published on 13th September 2005
Abstract
Upon complexation of a rectangular-shaped achiral macrocyclic host with chiral guests, twisting deformation occurs to induce exciton-type bisignated CD, whereas a chiral rectangular host undergoes a similar structural change only with the matching enantiomer of a chiral guest.
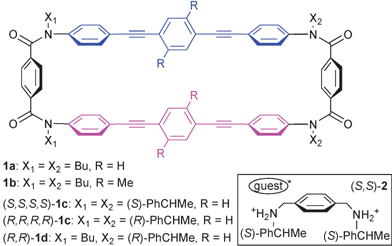
Molecular recognition is one of the main topics in host–guest chemistry.1 Chiral recognition is especially important in relation to biological systems where many neurotransmitters and hormones contain asymmetric centers. Inspired by the dynamic recognition systems in nature,2 we recently developed a terephthalamide-type exotopic host molecule.3 Upon complexation with catecholamine-type biogenic compounds, such as adrenaline,3b the host undergoes a drastic change in geometry with the formation of a supramolecular cyclophane structure by hydrogen bonding at amide carbonyl groups. For a terephthalamide unit incorporated into a cyclic structure in another host,4 the two carbonyl groups are preorganized in a syn arrangement, which is suitable for guest binding. We envisaged that a host with a larger-sized ring can adopt both rectangular and skewed shapes5 and may undergo dynamic molecular recognition thanks to interconversion between them upon complexation. Exoditopic hosts such as 1 are more promising in terms of homotropic allosteric effects, as shown in Scheme 1. When the direction of twisting is controlled6 by the asymmetric centers of the bidentate guest, such as (S,S)-2, a strong bisignated CD signal is generated upon complexation due to the exciton coupling7 of 1,4-bis(phenylethynyl)benzene (BPEB) chromophores (λmax 320 nm).8 We report here the chiral-sensing properties of newly designed macrocycles 1 based on their complexation-induced changes in conformation.
 | ||
| Scheme 1 | ||
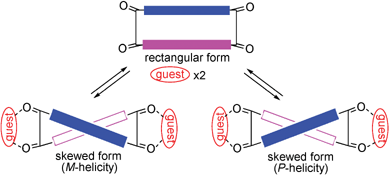
Exoditopic host 1a was prepared by the repeated Sonogashira coupling of N,N′-bis(4-iodophenyl)terephthalamide 4a (Scheme 2), which was obtained from terephthaloyl chloride and N-butyl-4-iodoaniline.9 Reaction of 4a with TMS-acetylene followed by deprotection gave bis(acetylene) 5a, which was then reacted with 1,4-diiodobenzene to give the bis(iodobenzene) derivative 6a. Pseudo high-dilution coupling between 5a and 6a gave the macrocyclic tetraamide 1a,‡ which was isolated as stable colorless crystals. When 2,5-diiodo-p-xylene was used in place of diiodobenzene, the host 1b‡ with four additional methyl groups could be prepared in a similar manner. The NMR spectrum of 1b exhibits a sharp singlet for these methyl groups, suggesting free rotation of the xylene moiety in 1b.
 | ||
| Scheme 2 | ||
When we started with 4c derived from chiral N-(1-phenylethyl)-4-iodoaniline, (R,R)- and (S,S)-6c were obtained following procedures similar to those for 6a. The double Sonogashira coupling between (S,S)-6c and its precursor (S,S)-5c gave the homochiral host (S,S,S,S)-1c.‡ Its enantiomer, (R,R,R,R)-1c,‡ was obtained from (R,R)-5c and (R,R)-6c. Macrocyclization between (R,R)-5c and achiral 6a gave (R,R)-1d,‡ in which only one of the two terephthalamide units possesses chiral auxiliaries. The optical rotations ([α]D23 in CHCl3) for (S,S,S,S)-1c, (R,R,R,R)-1c and (R,R)-1d are +1240, −1250 and −602°, respectively.
The X-ray analysis§ of achiral host 1a shows that it adopts a Ci-symmetric distorted rectangular form with approximate dimensions of 7.4 × 19.2 Å (separation between amide nitrogens) (Fig. 1). In the terephthalamide moiety, two carbonyl groups are arranged in the syn form (dihedral angle 1.0°) and the central phenylene group is rotated about 40° with respect to the amide units. The BPEB chromophores are arranged in parallel without any close intramolecular contacts. Since tetramethylated macrocycle 1b§ with a different packing arrangement adopts a nearly identical molecular shape, the observed rectangular form should not result from the packing force in the crystal, but rather must be the intrinsically favored geometry for the macrocycles prepared here.
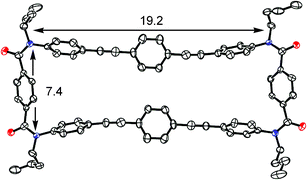 | ||
| Fig. 1 X-ray structure of achiral host 1a measured at −120 °C. | ||
A complexation study was performed with the achiral hosts 1a,b and the bidentate chiral guest (S,S)-2 containing two phenylethylammonium units. The p-xylene-α,α′-diyl unit in guest 2 is expected to provide the ammonium units with a suitable separation to bridge the terephthalamide moiety in 1. Upon portionwise addition of (S,S)-2 to a solution of 1b in CDCl3 (10−3 M), the terephthaloyl protons of the host exhibit a gradual upfield shift from 7.15 (0 equiv.) to 7.09 (2.2 equiv.) ppm. On the other hand, in the presence of 1.6 equiv. of 1b, the phenylene protons of (S,S)-2 (7.17 ppm) show a significant upfield shift of 0.50 ppm. These complexation-induced shifts (CISs) indicate the proximity of these aromatic units in the host–guest complex. Supramolecular paracyclophane formation through double hydrogen bonding at the shorter axes of 1 most likely accounts for this observation. Notable CISs were also found in the BPEB units, which are far from the binding sites for (S,S)-2. Small but significant downfield shifts of 0.02 ppm for the methyl protons in the middle of BPEB chromophores clearly show that complexation with (S,S)-2 induces a change in geometry of the macrocycle 1b, by which these methyl groups are deshielded by another BPEB unit.
To obtain more information on the complexation-induced structural changes,10 the complexation of 1a and (S,S)-2 was followed by an examination of the circular dichroism (CD) spectrum. Upon the gradual addition of (S,S)-2 to a solution of 1a (1.2 × 10−4 M) in CH2Cl2, a pair of negative (λext 345 nm; Δε −12) and positive (310, +10) Cotton effects were observed (Fig. 2). Such a bisignated CD (A = −22) is characteristic of the exciton coupling7 of the chromophores. Since the longest-wavelength absorption of 1a is assigned to that of the BPEB unit with a transition moment along the long axis,8 the observed negative couplet indicates twisting deformation of the macrocycle into a skewed conformation with predominant M-helicity6a,7 (Scheme 1). The sigmoidal curvature (Fig. 2, inset) for the relationship between the guest/host ratio and Δε indicates that the present complexation exhibits positive homotropic allosterism.11 In contrast, the monodentate guest (S)-3 [Ph-CHMe-NH2+-CH2-Ph (50 equiv.)] did not induce any couplet in the CD spectrum. The latter result indicates that the twisting deformation is caused not by simple binding with guests but by the formation of a supramolecular paracyclophane structure with the proper bidentate guests. The out-of-plane deviations of two carbonyl groups of 1a toward different directions upon complexation with 2 are responsible for twisting into the skewed conformation. These results show that bis(ammonium)-type chiral guest (S,S)-2 binds at each of the two terephthalamide moieties of macrocyclic host 1a to induce a change in geometry to a chiral skewed conformation, and (S)-phenylethyl auxiliaries on the guest bias the chiral sense of twisting in favor of M-helicity. Since the CD signal of (S,S)-2 is very weak (λext 261 nm; Δε +0.68), complexation with 1a results in much easier detection of (S,S)-2 with light of longer wavelength (“chiroptical enhancement”).
![Continuous changes in the CD spectrum upon complexation of achiral 1a
(1.2 × 10−4 M) with (S,S)-2 in CH2Cl2: a) 0.5 equiv., b) 1.0 equiv., c) 1.5 equiv., d) 2.0 equiv., e) 4.0 equiv. and f) 6.0 equiv. [inset] Sigmoidal relationship between Δε and the ratio of (S,S)-2/1a.](/image/article/2005/CC/b510134d/b510134d-f2.gif) | ||
| Fig. 2 Continuous changes in the CD spectrum upon complexation of achiral 1a (1.2 × 10−4 M) with (S,S)-2 in CH2Cl2: a) 0.5 equiv., b) 1.0 equiv., c) 1.5 equiv., d) 2.0 equiv., e) 4.0 equiv. and f) 6.0 equiv. [inset] Sigmoidal relationship between Δε and the ratio of (S,S)-2/1a. | ||
To construct a more advanced chiral sensing system based on the complexation-induced change in geometry of the macrocyclic host, complexation of the chiral host 1c with the chiral guest 2 was examined to see if the asymmetric auxiliaries on the macrocycle endow 1c with chiral recognition properties to exhibit a stereospecific change in conformation upon complexation. As shown in Scheme 3, only a matching enantiomer can induce a change in geometry to a skewed conformation that shows a strong couplet in the CD spectrum by exciton coupling. With the mismatched enantiomer, twisting deformation would not occur since the direction of twisting favored by the guest may differ from that favored by the chiral auxiliaries on the host.
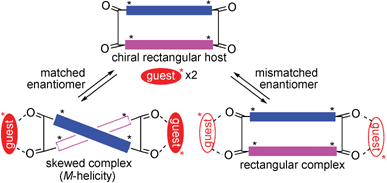 | ||
| Scheme 3 | ||
As shown in Fig. 3, the Cotton effect of the uncomplexed (S,S,S,S)-1c is positive over its entire absorption wavelength, and the CD spectrum of (R,R,R,R)-1c is its mirror image. Since (R,R)-1d exhibits a similarly shaped Cotton effect with half of its intensity,‡ the observed chiroptical properties are provided to the BPEB chromophores directly by the chiral auxiliaries on the periphery. Absence of a bisignated CD indicates that the asymmetric centers on the macrocycle do not cause the twisting deformation of the rectangular-shaped host 1c.
![[upper] Continuous changes in the CD spectrum upon complexation of (S,S,S,S)-1c
(1.4 × 10−4 M) with (S,S)-2 in CH2Cl2: a) 0 equiv., b) 2 equiv. and c) 4 equiv. [lower] Continuous changes in the CD spectrum upon complexation of (R,R,R,R)-1c
(1.1 × 10−4 M) with (S,S)-2 in CH2Cl2: d) 0 equiv., e) 2 equiv. and f) 4 equiv.](/image/article/2005/CC/b510134d/b510134d-f3.gif) | ||
| Fig. 3 [upper] Continuous changes in the CD spectrum upon complexation of (S,S,S,S)-1c (1.4 × 10−4 M) with (S,S)-2 in CH2Cl2: a) 0 equiv., b) 2 equiv. and c) 4 equiv. [lower] Continuous changes in the CD spectrum upon complexation of (R,R,R,R)-1c (1.1 × 10−4 M) with (S,S)-2 in CH2Cl2: d) 0 equiv., e) 2 equiv. and f) 4 equiv. | ||
With the admixture of (S,S,S,S)-1c (1 × 10−4 M) and (S,S)-2 (4 equiv.), a drastic change in geometry was suggested by a strong bisignated CD (λ = 328 nm, A = −70) (Fig. 3). The negative couplet indicates predominant M-helicity for the twisting deformation, as in the complexation between achiral 1a and (S,S)-2. On the other hand, similar complexation between (R,R,R,R)-1c and (S,S)-2 did not induce any bisignated CD, suggesting that the rectangular shape of the macrocycle is retained for the mismatched pair. These results demonstrate the outstanding chiral recognition properties of exoditopic host 1c, which exhibits a stereospecific response with 2 by providing a strong bisignated CD signal only with a matched chiral guest (“stereospecific chiroptical modulation”).
Complexation studies of 1c with neurotransmitters containing chiral benzylammonium units are now in progress to examine the possible applicability of the present motif as novel sensory systems.
Notes and references
- Recent reviews: (a) J. Rebek, Jr., Angew. Chem., Int. Ed., 2005, 44, 2068 CrossRef; (b) P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386 CrossRef CAS; (c) S. Shinkai and M. Takeuchi, Bull. Chem. Soc. Jpn., 2005, 78, 40 CrossRef CAS; (d) C. A. Hunter, Angew. Chem., Int. Ed., 2004, 43, 5310 CrossRef CAS; (e) W. Ong, M. Gómez-Kaifer and A. E. Kaifer, Chem. Commun., 2004, 1677 RSC; (f) F. Diederich, in Modern Cyclophane Chemistry, ed. R. Gleiter and H. Hopf, Wiley-VCH, Weinheim, 2004, pp. 519–546 Search PubMed.
- (a) D. E. Koshland, Jr., Angew. Chem., Int. Ed. Engl., 1994, 33, 2375 CrossRef; (b) M. Gerstein, A. M. Lesk and C. Chothia, Biochemistry, 1994, 33, 6739 CrossRef CAS.
- (a) H. Kawai, R. Katoono, K. Nishimura, S. Matsuda, K. Fujiwara, T. Tsuji and T. Suzuki, J. Am. Chem. Soc., 2004, 126, 5034 CrossRef CAS; (b) H. Kawai, R. Katoono, K. Fujiwara, T. Tsuji and T. Suzuki, Chem. Eur. J., 2005, 11, 815 CrossRef CAS.
- R. Katoono, H. Kawai, K. Fujiwara and T. Suzuki, Tetrahedron Lett., 2004, 45, 8455 CrossRef CAS.
- (a) P. N. W. Baxter, Chem.–Eur. J., 2003, 9, 2531 CrossRef CAS; (b) P. N. W. Baxter and R. Dali-Youcef, J. Org. Chem., 2005, 70, 4935 CrossRef CAS.
- (a) B. Köhler, V. Enkelmann, M. Oda, S. Pieraccini, G. P. Spada and U. Scherf, Chem.–Eur. J., 2001, 7, 3000 CrossRef CAS; (b) Y. Tanaka, H. Katagiri, Y. Furusho and E. Yashima, Angew. Chem., Int. Ed., 2005, 44, 3867 CrossRef CAS.
- N. Berova, K. Nakanishi and R. W. Woody, Circular Dichroism: Principles, Applications and Latest Advances, 2nd edn., Wiley-VCH, New York, 2000 Search PubMed.
- M. Levitus, K. Schmieder, H. Ricks, K. D. Shimizu, U. H. F. Bunz and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2001, 123, 4259 CrossRef CAS.
- K. Okano, H. Tokuyama and T. Fukuyama, Org. Lett., 2003, 5, 4987 CrossRef CAS.
- (a) P. Comba, A. Kühner and A. Peters, J. Chem. Soc., Dalton Trans., 1999, 509 RSC; (b) Y. Mizuno and T. Aida, Chem. Commun., 2003, 20 RSC; (c) M. A. Mateos-Timoneda, M. Crego-Calama and D. N. Reinhoudt, Chem. Soc. Rev., 2004, 33, 363 RSC.
- M. Takeuchi, M. Ikeda, A. Sugasaki and S. Shinkai, Acc. Chem. Res., 2001, 34, 865 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: spectral data and synthetic procedures for the new compounds [1a, 1b, (S,S,S,S)/(R,R,R,R)-1c, (R,R)-1d, (S,S)-2, (S)-3, 4a, (S,S)/(R,R)-4c, 5a, (S,S)/(R,R)-5c, 6a, 6b and (S,S)/(R,R)-6c]. See http://dx.doi.org/10.1039/b510134d |
| ‡ Electronic and CD spectral data in CH2Cl2 are as follows. 1a: λmax/nm 317 (log ε 4.99); 1b: λmax/nm 317 (log ε 4.96); (R,R,R,R)-1c: λmax/nm 306 (log ε 4.98), λext/nm 333 (Δε −68.1), 311 (−48.8), 291 (−70.4), 267 (−6.9), 249 (−67.4); (S,S,S,S)-1cλext/nm 333 (Δε +68.3), 313 (+49.0), 290 (+71.0), 267 (+8.0), 251 (+67.0); (R,R)-1d: λmax/nm 313 (log ε 4.95), λext/nm 337 (Δε −30.1), 318 (−16.1), 299 (−28.9), 269 (+0.7), 250 (−26.2); (S,S)-2 (BF4−)(BAr4−) [Ar = 3,5-(CF3)2C6H3]: λmax/nm 274 (log ε 3.59), 269 (3.70), 261 (3.65), λext/nm 268 (Δε +0.61), 261 (+0.68), 255 (+0.48); (S)-3 BF4−: λmax/nm 268 (log ε 2.49), 262 (2.69), 257 (2.68), λext/nm 268 (Δε +0.18), 261 (+0.21), 255 (+0.13). |
| § Crystal data of 1a: MF C76H68N4O4, FW 1101.40, monoclinic P21/n, a = 18.788(4), b = 8.976(2), c = 18.881(4) Å, β = 90.193(6)°, V = 3184.0(12) Å3, ρ (Z = 2) = 1.149 g cm−3, T = 153 K, R = 11.2%. Crystal data of 1b·AcOEt solvate: MF C88H92N4O8, FW 1333.72, monoclinic C2/c, a = 33.738(7), b = 8.957(2), c = 27.599(6) Å, β = 111.546(3)°, V = 7758(3) Å3, ρ (Z = 4) = 1.142 g cm−3, T = 153 K, R = 8.3%. CCDC 279787–279788. See http://dx.doi.org/10.1039/b510134d for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |