A furanosyl-carbonate autoinducer in cell-to-cell communication of V. harveyi†
Kathleen M.
McKenzie
,
Michael M.
Meijler
,
Colin A.
Lowery
,
Grant E.
Boldt
and
Kim D.
Janda
*
The Skaggs Institute for Chemical Biology and Departments of Chemistry and Immunology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA. E-mail: kdjanda@scripps.edu
First published on 6th September 2005
Abstract
An autoinducer arising from reaction of cyclized S-DPD and carbonate is shown to induce light in V. harveyi and thus may play a previously unknown role in quorum sensing.
Quorum sensing is used by bacteria as a means to rapidly coordinate gene expression patterns in response to environmental cues.1 Quorum sensing can be used to control such functions as motility, conjugation, competence, sporulation, virulence and biofilm formation.2 The mechanism of this process is based on the secretion and binding of signaling molecules, called autoinducers, the bulk of which can be classified into two categories. Typically, Gram-negative bacteria communicate using acylated homoserine lactones (AHLs) while Gram-positive bacteria utilize oligopeptide autoinducers.3 However, this simplistic categorization is constantly being expanded as other small molecule autoinducers are identified. The Gram-negative pathogenic bacterium Pseudomonas aeruginosa exploits the Pseudomonas quinoline signal (PQS), in addition to AHLs, to control virulence.4 The Gram-positive soil bacterium Streptomyces makes use of γ-butyrolactones to regulate morphological differentiation and secondary metabolite production.5 The plant pathogen Ralstonia solanacearum utilizes 3-hydroxypalmitic acid methyl ester (3-OH PAME).6 The nodulation genes of Bradyrhizobium japonicum are controlled by the small molecule bradyoxetin.7 One unique autoinducer, AI-2, has been identified in a wide variety of Gram-negative and -positive bacteria and has been suggested to be a universal species-nonspecific signaling molecule.8
First identified in the marine bacterium Vibrio harveyi for its ability to control bioluminescence, AI-2 is believed to be generated by conversion of the ribose moiety of S-ribosylhomocysteine into (4S)-4,5-dihydroxy-2,3-pentanedione (S-DPD) by the protein LuxS.8a Recently, the structure of this molecule was determined, as bound to the receptor protein LuxP, to be a furanosyl borate diester formed from reaction of the ring closed isomer of DPD and boric acid (Scheme 1).9 Although unusual, as boron has not typically been shown to play a role in biological systems, boron is present in seawater at a concentration of approximately 0.4 millimolar thereby accounting for the furanosyl borate diester.10
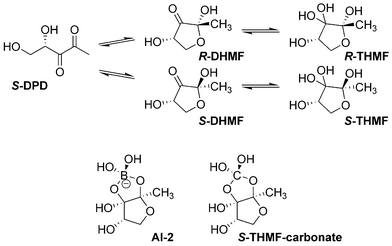 | ||
| Scheme 1 S-DPD and related biological structures. | ||
Although boron may be highly relevant to marine bacteria, it is probably not essential to other forms of bacteria, therefore we sought to further investigate the necessity of boron in interspecies signaling. Indeed, during the course of our studies, a report was published indicating that Salmonella typhimurium does not require boron for signaling and instead recognizes (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (R-THMF), a hydrated furanosyl form of the precursor DPD (Scheme 1).11
The first critical question addressed was whether boron is the only metal capable of complexing DPD and therefore inducing light expression in V. harveyi. To assess their potential biological activity, a variety of metal salts were chosen and screened at an initial concentration of 1 mM for their ability to induce light production in V. harveyi strain BB170 using a protocol reported by Schauder et al.12 As shown in Fig. 1, caesium carbonate and calcium chloride both increased light production in autoinducer bioassay (AB) medium while magnesium chloride and iron salts showed no effect. However, when assayed in boron-free (BFAB) medium, only caesium carbonate was able to restore light production. Iron appeared to have some effect on light production in boron-free AB medium, but since iron is known to increase bacterial growth, this result may be an artifact of increased avidity, as opposed to signaling.13
 | ||
| Fig. 1 Initial screen of metal salts at a concentration of 1 mM for their ability to induce light production in V. harveyi strain BB170, in the presence (AB) or absence (BFAB) of boron. (a) No additive; (b) B(OH)3; (c) CaCl2; (d) Cs2CO3; (e) MgCl2; (f) MnCl2; (g) FeSO4; (h) FeCl3. | ||
To further understand the potential role of caesium in AI-2 signaling and to allow for comparison with the previously tested chloride salts, caesium chloride was tested for its ability to induce light production. Interestingly, caesium chloride had no effect in boron containing medium and was unable to induce light in boron-free medium. Neither metal salt altered the pH of the medium or the growth of the bacteria. When examining the differences between caesium carbonate and caesium chloride, it became apparent that the main difference was the bond strength of the caesium–oxygen bond versus the caesium–chloride bond, the former being stronger due to more ionic character.14
We investigated metal salts with more labile metal–oxygen bonds, expecting induction of light, and if this were the case, then the converse would also be true. The metal chlorides were reacted with equimolar amounts of silver triflate, causing the formation of metal triflates, with silver chloride precipitating from solution. These solutions were tested for their ability to induce light production, however once again no effect was observed in boron-free medium. Additionally, when sodium chloride was added to caesium carbonate, no inhibition of light production was observed. Thus it was concluded that the positive effect of signaling is likely contributed to the carbonate species, rather than the metal ion, through the formation of an orthocarbonate structure (2S,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran-carbonate (S-THMF-carbonate) shown in Scheme 1. Interestingly, this compound was among the structures initially considered for AI-2 based on the crystal structure of AI-2 bound to its receptor LuxP, but ultimately dismissed because of its inherent instability.9
Several water soluble carbonates, namely potassium, sodium and ammonium carbonate, were serially diluted and assayed for their ability to induce light. Gratifyingly, when added to V. harveyi strain BB170 cells in boron-free medium, they all induced bioluminescence, as shown in Fig. 2. The effective concentration of boric acid is approximately 10 µM while caesium carbonate and potassium carbonate have an effective concentration approximately 50-fold higher.‡ Sodium carbonate and ammonium carbonate exhibit activity at concentrations greater than 1 mM, presumably due to smaller cation size. The smaller sodium cation is more tightly associated to the carbonate, thus requiring more energy for its dissociation.15
 | ||
| Fig. 2 V. harveyi strain BB170 light induction with increasing concentrations of boric acid or metal carbonates. EC50 (µM) ± SD: B(OH)3 10.6 ± 3.0; Cs2CO3 478 ± 55; K2CO3 569 ± 67; Na2CO3 ≥ 1000; (NH4)2CO3 ≥ 1000. | ||
Alternatively, when 0.5 mM solutions of either boric acid or the various carbonates were added to serial dilutions of DPD, light was induced in V. harveyi strain MM30 (a LuxS mutant that is unable to synthesize AI-2), as shown in Fig. 3.16 In this case, AI-2 is active at 48 nM while the presumed orthocarbonate species, S-THMF-carbonate, is 2- to 3-fold less active. We have attempted to characterize the S-THMF-carbonate species by NMR, mass spectral analysis and IR, however, the orthocarbonate appears to be highly unstable in solution and in all probability is in a continuous flux of association/dissociation, which likely contributes to its lower activity. Once again some variation in activity is observed with the different carbonates, presumably due to the differences in solvation energy and bond dissociation of the counter ions. The 50-fold difference in signaling observed between boron and the carbonates (Fig. 2) is due to the inherent instability of the proposed orthocarbonate species. In order to overcome this instability, a high concentration of carbonate must be present. However, once formed, the orthocarbonate acts as a signaling molecule with an activity very close to that of AI-2 (Fig. 3) due to its analogous structure and potential for binding similarly into the active site of LuxP.
 | ||
| Fig. 3 V. harveyi strain MM30 light induction at 0.5 mM boric acid or carbonates with increasing concentrations of synthetic DPD. EC50 ± SD (nM): B(OH)3 48 ± 5; Cs2CO3 108 ± 7; K2CO3 140 ± 12; Na2CO3 134 ± 6; (NH4)2CO3 161 ± 9. | ||
While investigating the role of various metals in AI-2 signaling, bioluminescence in V. harveyi was observed as a result of addition of metal carbonates, leading to the conclusion that a hitherto unknown autoinducer S-THMF-carbonate, arising from the reaction of a furanosyl form of S-DPD with carbonate, plays a role in V. harveyi quorum sensing. Although our attempts to obtain biophysical data on the orthocarbonate species have been unsuccessful due to inherent instability, we feel this result is defensible for several reasons. First, S-THMF-carbonate fits perfectly in the binding pocket of LuxP and was initially considered as a plausible structure of AI-2.9 Secondly, carbonate is present in sea water at a concentration of 0.2 millimolar making S-THMF-carbonate a reasonable autoinducer for the marine species V. harveyi.17 Lastly, when studying Streptococcus gordonii, a bacterium in dental biofilms, Blehert and coworkers found that it was necessary to add sodium carbonate at high concentrations to observe light production in the V. harveyi autoinducer bioassay.18 As bicarbonate is the primary buffering agent in saliva, one could hypothesize that S. gordonii is utilizing primarily S-THMF-carbonate as an autoinducer. This work provides evidence for an additional signal molecule in the ever expanding family of universal autoinducers derived from the simple precursor S-DPD, showing just how elegantly the language of bacterial communication has evolved.
Notes and references
- J. W. Hastings and K. H. Nealson, Annu. Rev. Microbiol., 1977, 31, 549–595 CrossRef CAS.
- S. Schauder and B. L. Bassler, Genes Dev., 2001, 15, 1468–1480 CrossRef CAS.
- G. J. Lyon and T. W. Muir, Chem. Biol., 2003, 10, 1007–1021 CrossRef CAS; C. Fuqua, S. C. Winans and E. P. Greenberg, Annu. Rev. Microbiol., 1996, 50, 727–751 CrossRef CAS; A. D. Grossman, Annu. Rev. Genet., 1995, 29, 477–508 CrossRef CAS; T. R. de Kievit and B. H. Iglewski, Infect. Immun., 2000, 68, 4839–4849 CrossRef CAS.
- E. C. Pesci, J. B. J. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg and B. H. Iglewski, Proc. Natl. Acad. Sci. USA, 1999, 96, 11229–11234 CrossRef CAS.
- K. F. Chater and S. Horinouchi, Mol. Microbiol., 2003, 48, 9–15 CrossRef CAS.
- A. B. Flavier, S. J. Clough, M. A. Schell and T. P. Denny, Mol. Microbiol., 1997, 26, 251–259 CrossRef CAS.
- J. Loh, R. W. Carlson, W. S. York and G. Stacey, Proc. Natl. Acad. Sci. USA, 2002, 99, 14446–14451 CrossRef CAS.
- (a) B. L. Bassler, M. Wright, R. E. Showalter and M. R. Silverman, Mol. Microbiol., 1993, 9, 773–786 CrossRef CAS; (b) B. L. Bassler, M. Wright and M. R. Silverman, Mol. Microbiol., 1994, 13, 273–286 CrossRef CAS.
- X. Chen, S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler and F. M. Hughson, Nature, 2002, 415, 545–549 CrossRef CAS.
- H. J. M. Bowen, in Trace Elements in Biochemistry; Academic:London, 1966 Search PubMed.
- S. T. Miller, K. B. Xavier, S. R. Campagna, M. E. Taga, M. F. Semmelhack, B. L. Bassler and F. M. Hughson, Mol. Cell, 2004, 15, 677–687 CrossRef CAS.
- S. Schauder, K. Shokat, M. G. Surette and B. L. Bassler, Mol. Microbiol., 2001, 41, 463–476 CrossRef CAS.
- C. M. Litwin and S. B. Calderwood, Clin. Microbiol. Rev., 1993, 6, 137–149 CAS.
- J. A. Kerr, in CRC Handbook of Chemistry and Physics 1999–2000: A Ready-Reference Book of Chemical and Physical Data; 81st edn.; CRC Press: Boca Raton, FL, 2000 Search PubMed.
- F. A. Cotton and G. Wilkinson, in Advanced Inorganic Chemistry: A Comprehensive Text; John Wiley & Sons, Inc.: New York, NY, 1980 Search PubMed.
- M. G. Surette, M. B. Miller and B. L. Bassler, Proc. Natl. Acad. Sci. USA, 1999, 96, 1639–1644 CrossRef CAS.
- T. Tyrell and R. E. Zeebe, Geochim. Cosmochim. Acta, 2004, 68, 3521 CrossRef.
- D. S. Blehert, R. J. Palmer, Jr., J. B. Xavier, J. S. Almeida and P. E. Kolenbrander, J. Bacteriol., 2003, 185, 4851–4860 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental details of bioluminescence assays. See http://dx.doi.org/10.1039/b509396a |
| ‡ A decrease in light production is always observed at high concentrations of boric acid. |
| This journal is © The Royal Society of Chemistry 2005 |
