Amplifying fluorescent polymer sensors for the explosives taggant 2,3-dimethyl-2,3-dinitrobutane (DMNB)†
Samuel W.
Thomas III
,
John P.
Amara
,
Rebekah E.
Bjork
and
Timothy M.
Swager
*
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, USA. E-mail: tswager@mit.edu; Fax: +1 617-253-7929; Tel: +1 617-253-4423
First published on 17th August 2005
Abstract
Structural and electronic effects on the efficiency of DMNB detection with fluorescent conjugated polymers are described.
Sensory devices based on amplified fluorescence quenching of solid-state conjugated polymer (CP) films can be highly sensitive, due to the amplification that arises from delocalized excitons sampling many potential binding sites within one excited-state lifetime.1 Our research group and others have demonstrated highly sensitive detection schemes using these amplifying fluorescent polymers (AFPs) for a number of analytes in solution and vapor phase.2 The best developed vapor phase application of this method has been the ultratrace detection of nitroaromatics often associated with high explosives such as 2,4,6-trinitrotoluene (TNT).3 The sensitivity of the resulting sensors is comparable to that of trained canines. The transduction mechanism is photoinduced charge transfer (PICT) from the polymer donor to the nitroaromatic that binds via a tight π-complex to the CP.
Although nitroaromatics are present in many explosives, present day security is in need of systems capable of matching comprehensive vapor phase detection of all high explosives such as can be achieved by canines. Toward this end we report the design of AFPs for the detection of the taggant 2,3-dimethyl-2,3-dinitrobutane (DMNB), which is a required additive in all legally manufactured plastic explosives. The detection of DMNB vapor has been previously demonstrated using ion mobility spectrometry, a reliable but less sensitive technique relative to AFPs.4 Although it has a relatively high vapor pressure of approximately 2.7 ppm (2.07 × 10−3 Torr at 25 °C),5 DMNB presents several challenges for vapor phase detection by AFPs. These include a relatively unfavorable reduction potential of approximately −1.7 V (vs. standard calomel electrode, SCE)6 and a highly three-dimensional structure that offers no opportunity to engage in π-stacking with a CP film.
In search of optimally sensitive and operationally useful AFPs for the detection of DMNB, we set out to design electronic structures that will have greater photoreduction abilities. Fig. 1 illustrates the qualitative energy level diagrams of the materials used in these experiments. Table 1 lists the Stern–Volmer constants [k(sv)] and quenching rate constants [k(q)] from Stern–Volmer experiments with these AFPs using DMNB as the quencher.7 The variation in the quenching efficiency of the poly(phenylene ethynylene) AFPs (PPEs, P1–3) reveals that the factors controlling the quenching rate constants are multifaceted. Although the π-donation of the amines reduces the ground state oxidation potential for P3, it also lowers the band gap and thereby increases P3's excited-state oxidation potential. The higher quenching efficiency of P2, with only one amine per repeat unit, is likely due to a balance between these two effects. All of the PPEs investigated showed very poor quenching rate constants, with even the best quenching rate constant falling an order of magnitude less than the diffusion controlled limit.
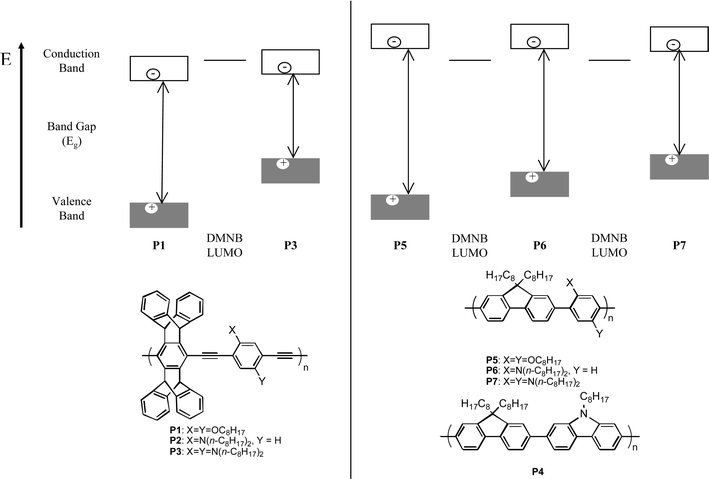 | ||
| Fig. 1 Qualitative band diagram of PPEs (left) and PPs (right) studied for sensitivity to DMNB. The twisted backbone of the PPs leads to a higher energy conduction band and allows for photoinduced electron transfer quenching. | ||
| Polymer | λ(em)/nm | τ(f)/ns | k(sv)/M−1 | k(q)/109 M−1 s−1 |
|---|---|---|---|---|
| P1 | 460 | 0.6 | ∼0 | <1 |
| P2 | 475 | 1.8 | 3.4 | 1.9 |
| P3 | 580 | 3.9 | <2 | <1 |
| P4 | 416 | 0.5 | 9.2 | 18 |
| P5 | 413 | 0.6 | 9.0 | 15 |
| P6 | 452 | 1.9 | 13 | 6.9 |
| P7 | 512 | 4.2 | 22 | 5.2 |
In order to decrease the excited-state oxidation potential and thereby improve the efficacy of the quenching reaction, larger band gap poly(phenylene)s (PPs) were prepared. PPs are optically blue-shifted relative to PPEs, due to a non-planar ground state conformation with decreased conjugation.8 All of the PPs investigated (Fig. 1) gave quenching rate constants significantly higher than P2, the PPE most efficiently quenched by DMNB. This supports our hypothesis that larger band gaps, and therefore higher energy conduction bands, will produce superior performance relative to analogous PPEs. In fact the most blue-shifted PPs are quenched by DMNB with diffusion controlled rate constants. As would be expected from simple orbital mixing arguments the aryl amines mainly raise the valence band (HOMO) levels and have a smaller perturbation on the conduction band (LUMO) energies, which lowers the band gaps for both PPEs and PPs.9 The PPs' wider band gap enables more exergonic electron transfer from the higher energy conduction bands to the DMNB LUMO.
Satisfying the energetic constraints of electron transfer quenching is a necessary, but not a sufficient condition for the trace detection of vapor phase analytes with solid-state AFPs. The degree of fluorescence quenching in the solid-state is also dependent upon other factors, such as the vapor pressure of the analyte, its binding constant to the fluorescent polymer film, and the mobility of excitons in the solid AFP. These usually have little connectivity with the quenching efficiency in solution, thereby limiting the amount of applicable information solution quenching experiments can supply for solid-state chemosensing systems.
Therefore, thin films of selected AFPs were simultaneously irradiated and exposed to equilibrium vapor pressures of DMNB. These experiments were carried out on a commercial Fido™ instrument manufactured by Nomadics Inc.,10 which continuously monitors the total fluorescence during vapor sampling. Experimentation showed the PPEs' solid-state fluorescence quenching to be very weak, with only P2 giving a 4–5% fluorescence attenuation in the presence of DMNB vapor. Hence, we have focused on the more promising PPs. In order to probe the effect of analyte molecular shape, benzophenone was also investigated as a vapor phase quencher. Benzophenone has a reduction potential (−1.6 V vs. SCE) similar to that of DMNB as well as a similar equilibrium vapor pressure (1.93 × 10−3 Torr at 25 °C).11 In contrast to DMNB, the flat structure of benzophenone could allow for strong π-stacking with the polymer film.
Fig. 2 and 3 present representative fluorescence traces obtained upon exposure of a thin film of P5 to either benzophenone or DMNB. Table 2 summarizes the results of the solid-state quenching experiments. The percentage of quenching due to equilibrium benzophenone vapor correlates well with the electron density on the polymer chain, with the diamine-containing P7 showing an average response of 60% quenching under constant flow. These results are consistent with strong polymer–analyte π-stacking, since polymers with more electron-rich π-systems will have a higher affinity for the more planar benzophenone. The larger binding affinity of benzophenone is also revealed in its temporal response in the sensor. The sensor response in Fig. 2 reveals that at short exposures DMNB is highly reversible indicating a weak binding (rapid off-rate). 20 s exposures to either benzophenone or DMNB (Fig. 3) are illustrative of the slower recovery times associated with benzophenone that indicate stronger binding constants with the AFPs. The larger binding affinity of benzophenone for the very electron-rich P7vs.P6 or other, less-electron rich polymers is large enough to outweigh any other factors that might make P7 less efficient, such as a smaller driving force for electron transfer or decreased exciton mobility.
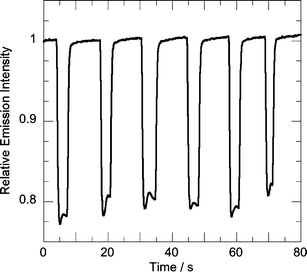 | ||
| Fig. 2 Emission intensity response of a film of P5 to repeated variable time exposures to equilibrium DMNB vapor. | ||
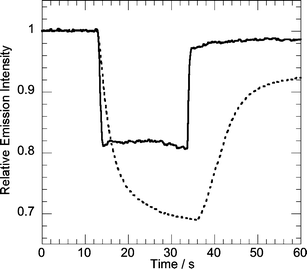 | ||
| Fig. 3 ∼20 s exposure of a film of P5 to equilibrium vapor of either DMNB (solid line) or benzophenone (dotted line). | ||
| Polymer | Benzophenone | DMNB |
|---|---|---|
| P4 | 5 ± 1% | 6 ± 1% |
| P5 | 29 ± 3% | 20 ± 5% |
| P6 | 38 ± 3% | 12 ± 3% |
| P7 | 58 ± 7% | 4 ± 1% |
The data in Table 2 show that no such simple structure–property relationship exists for quenching by DMNB. The moderately electron-rich polymer P5 is the superior material, giving a highly reversible and rapid quenching response of about 20%. More electron-rich and more electron-poor polymers give smaller responses. This is indicative of several competing factors contributing to the observed results. Electrostatic attraction is certainly a factor in the solid-state detection of DMNB, since on an absolute scale it is an electron-deficient compound. However, with no prospects for strong π-stacking, DMNB's binding differences appear to have little to do with the electron density in the π-system.
Other parameters that can affect a particular material's quenching efficiency in response to an analyte vapor include the rate of PICT, which was demonstrated in the solution experiments to be smaller for the amine-substituted PPs. In addition, the mobility of the excitons in films of P6 and P7 may be substantially smaller than for P5. The large Stokes shift and excited-state lifetimes of these materials are indicative of a substantial structural rearrangement in the excited state.7 The inability of certain polymer chains to adopt the preferred conformation in the solid-state and the activated nature of conformational changes will effectively impose a “viscous drag” and produce excitons with a higher effective mass.12
It is clear that the efficient detection of very weakly binding analytes relies on the balance of multiple parameters that will likely be somewhat different for each target analyte. P5 offers a good balance of PICT driving force, electrostatic attraction, and exciton mobility to give a reversible and rapid response to DMNB vapor. In addition, P5, which has previously been investigated for potential use as blue emitter in PLEDs,13 is highly emissive in the solid-state and shows good photochemical stability, especially compared to the amine-containing PPs P6 and P7.
DMNB is also known to undergo a reductive cleavage reaction with cathodic electrolysis, one of the products of which is the nitrite ion.6 We have performed colorimetric tests to confirm the photochemical generation of nitrite in the quenching reactions with our polymers. The generation of this product is unique to DMNB relative to other quenchers such as TNT. It may be possible to incorporate confirmatory chemistry into a sensory device to discriminate DMNB from other potential quenchers.
In conclusion, we have reported amplifying fluorescent polymers for the trace vapor detection of the plastic explosive taggant DMNB. A variety of polymer structures were investigated for their efficacy in detecting DMNB. Solution Stern–Volmer experiments revealed that as a group, the excited states of poly(phenylene)s, due to their wider band gaps, have larger PICT driving forces to DMNB than the inefficient PPEs. Solid-state quenching experiments show that DMNB's highly three-dimensional shape and lack of π-stacking with polymer films only allows for weak, but sufficient binding to allow for its vapor phase detection by amplified fluorescence quenching. Further studies will focus on improving the ability of AFPs to detect challenging analytes, especially including the incorporation of strong hydrogen bond donors to increase the binding affinity of these analytes to AFPs.
We acknowledge the generous financial support of the Transportation Security Administration, Sandia National Laboratories, and the Technical Support Working Group.
Notes and references
- Q. Zhou and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 12593 CrossRef CAS.
- (a) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS; (b) T. M. Swager, Acc.. Chem. Res., 1998, 31, 201 CrossRef CAS.
- (a) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 5321 CrossRef CAS; (b) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 12389.
- (a) J. M. Perr, K. G. Furton and J. R. Almirall, J. Sep. Sci., 2005, 28, 177 Search PubMed; (b) R. G. Ewing and C. J. Miller, Field Anal. Chem. Technol., 2001, 5, 215 Search PubMed.
- Containing the Threat from Illegal Bombings: An Integrated National Strategy for Marking, Tagging, Rendering Inert, and Licensing Explosives and Their Precursors, National Academy Press, Washington DC, 1998, p. 47 Search PubMed.
- W. J. Bowyer and D. H. Evans, J. Org. Chem., 1988, 53, 5234 CrossRef CAS.
- The synthesis of these materials has been described. See ref. 3b and (a) S. W. Thomas III and T. M. Swager, Macromolecules, 2005, 38, 2716 CrossRef; (b) J.-F. Morin and M. Leclerc, Macromolecules, 2001, 34, 4680 CrossRef CAS.
- J. S. S. Lamba and J. M. Tour, J. Am. Chem. Soc., 1994, 116, 11723 CrossRef CAS.
- J. L. Brédas, D. A. dos Santos, C. Quattrocchi, R. H. Friend and A. J. Heeger, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1994, 35, 185 CAS.
- C. Cumming, C. Aker, M. Fisher, M. Fox, M. laGrone, D. Reust, M. Rockley, T. M. Swager, E. Towers and V. Williams, IEEE Trans. Geosci. Remote Sens., 2001, 39, 1119 CrossRef.
- Handbook of Physical Properties of Organic Chemicals, ed. P. H. Howard and W. M. Meylan, CRC Press, Boca Raton, 1997 Search PubMed.
- Excitons are often described as quasiparticles.
- W.-L. Yu, J. Pei, Y. Cao, W. Huang and A. J. Heeger, Chem. Commun., 1999, 1837 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Spectra and Stern–Volmer plots from solution quenching experiments. See http://dx.doi.org/10.1039/b508408c |
| This journal is © The Royal Society of Chemistry 2005 |
