A two-dimensional clathrate hydrate sandwiched by planar arrays of a copper complex†
Riichi
Miyamoto
a,
Rika Tanaka
Hamazawa
a,
Masakazu
Hirotsu
a,
Takanori
Nishioka
a,
Isamu
Kinoshita
*a and
L. James
Wright
*b
aDepartment of Molecular Materials Science, Osaka City University, 3-3-138 Sugimoto, Osaka 558-8585, Japan. E-mail: isamu@sci.osaka-cu.ac.jp; Fax: +81-6-6690-2753; Tel: +81-6-6605-2546
bDepartment of Chemistry, University of Auckland, Private Bag 92019, Auckland 1020, New Zealand. E-mail: lj.wright@auckland.ac.nz; Fax: +64-9-373-7422; Tel: +64-9-373-7599 ext 88257
First published on 15th June 2005
Abstract
The first structurally characterised example of a 2-D clathrate hydrate spontaneously assembles when [CuF(tptm)] (1) (tptm = tris(2-pyridylthio)methyl) crystallises from toluene/water solutions to produce a material in which planar arrays of [CuF(tptm)] sandwich and hydrogen bond to continuous 2-D sheets of water that incorporate toluene molecules at regular intervals.
Studies of the water–water and water–solute interactions in water confined to extremely small spaces are relevant to the understanding of bulk water1 as well as important processes in biology,2 materials science3 and geology.4 One experimental approach that provides insight into these extreme water environments involves structural investigations of crystalline hosts containing small water clusters,5 1-D water chains or tapes6 or 2-D sheets of water molecules.7 Here we report the crystal structures of two closely related materials that both contain planar arrays of the amphiphilic copper complex [CuF(tptm)] (1), as well as planar 2-D sheets of water. In crystalline 4[CuF(tptm)]·12(H2O) (2), each continuous water sheet is sandwiched between two planar arrays of [CuF(tptm)] molecules. The [CuF(tptm)] molecules are oriented with all the fluoride groups directed towards, and hydrogen bonded with, the sandwiched water sheet. Remarkably, the same composite arrangement is retained in crystalline 6[CuF(tptm)]·14.5(H2O)·0.5(toluene) (3), but in this case toluene molecules are incorporated at regular intervals within each of the water sheets. To our knowledge, this latter material is the first structurally characterised example of a 2-D clathrate hydrate.
We have recently engaged in an extensive research programme aimed at studying the chemistry of metal complexes with ligands based on the heterocyclic group, pyridine-2-thiolate, as well as the supramolecular architectures these materials form in the solid state.8 While investigating the chemistry of [Cu(tptm)(CH3CN)]PF6, which contains a novel CuII–C(sp3) bond, we found that treatment with fluoride ion produces the neutral complex 1. This complex is amphiphilic in character and is soluble in both toluene and water. On crystallisation of 1 from dichloromethane/cyclohexane solutions that contain traces of water, crystals of 2 are formed (Scheme 1).
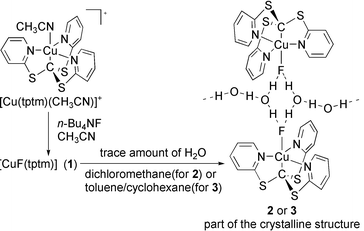 | ||
| Scheme 1 Syntheses of 1–3. | ||
The X-ray crystal structure‡ of 2 reveals that there are four independent complex molecules and 12 independent water molecules in the asymmetric unit. On viewing the extended structure, it is immediately apparent that the molecules of 1 are arranged in two dimensional, planar arrays (see Fig. 1a). Within each array all the Cu–F bonds are oriented nearly perpendicular to the plane of the array and all the fluorides point in the same direction. The maximum deviation of the copper atoms from the mean plane through all the copper atoms in an array is 0.0238(3) Å. The orientation of the three pyridyl groups of each molecule of 1 is almost perpendicular to the plane of the array and the molecules of 1 mesh together as shown in Fig. 2. In every molecule of 1, two of the pyridine rings are each involved in one offset-face-to-face (OFF) and one edge-to-face (EF) intermolecular interaction, while the third pyridine ring is involved in two edge-to-face intermolecular interactions. Thus, the planar arrays of 1 can be viewed as continuous networks of the (OFF)(EF)2 embrace motifs.9 The intermolecular distances between the pairs of pyridine rings involved in the OFF interactions range from 3.6(3) to 4.2(5) Å.
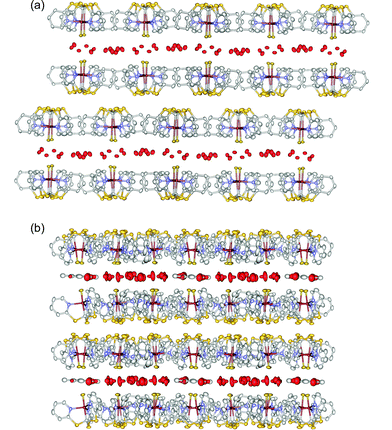 | ||
| Fig. 1 (a) Side-on view of the layered structure of 2 along the a axis and (b) of 3 along the c axis showing how each 2-D water sheet is sandwiched by two planar arrays of complex 1. Water molecules are coloured red and both sulfur and fluorine atoms are coloured yellow. | ||
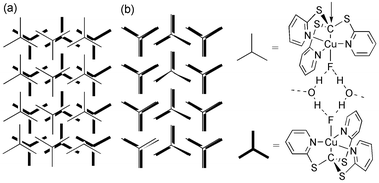 | ||
| Fig. 2 Schematic representation showing the relative position of each copper atom (with its three coordinated pyridyl groups) within the two planar arrays of 1 that sandwich each water layer in (a) 2 and (b) 3 when viewed from above (i.e. perpendicular to the ab plane for 2 and perpendicular to the bc plane for 3). | ||
The arrays of molecules of 1 are arranged in pairs, with all the fluoride ligands directed inwards towards each other. Sandwiched between each of these 2-D double-layers is a relatively flat, continuous, 2-D layer, or sheet, of water molecules. The extended crystal lattice can be viewed as being built up of stacks of these composite sheets. The closest contacts between atoms in adjacent composite sheets are large (2.64(5) Å for the shortest S to H(pyridine) distance) and van der Waals interactions are expected to be an important factor in holding the composite sheets together, as is the case in some metal sulfides.
As might be expected, the fluorides form hydrogen bonds to the water sheet and half of the water molecules are involved in these interactions. Two different types of hydrogen bonding arrangements between fluoride and water are observed. There are equal numbers of each type of arrangement and for each arrangement there are two distinct sites that differ slightly in bond distances and geometries. In one arrangement two closely approaching fluorides (average separation, 4.10(2) Å) are bridged by a pair of water molecules to form a tilted, 4-membered ring with OH⋯F distances averaging 2.692(9) Å. In the other arrangement a pair of fluorides that have a larger separation (average distance, 4.30(3) Å) bridge three water molecules with considerably longer OH⋯F distances (average, 2.78(3) Å). In combination, these hydrogen bonds are expected to play a key role in directing the formation of the composite sheets and also in determining the observed 3.92(4) Å separation between the two “slipped” arrays.
The water layer is comprised of a continuous sheet of fused 5- and 6-membered rings of water molecules, with the continuity of each ring disrupted at one point by bridging fluoride. From X-ray crystallography it was possible to identify the location of all the hydrogen atoms, and hence define the hydrogen-bonding network formed within the sheet. Each hydrogen atom participates in a hydrogen bonding interaction with either oxygen or fluorine, and a view of the water sheet illustrating the hydrogen-bonding network is provided in Fig. 3a. The water layer is quite planar with the maximum oxygen atom deviation from the mean plane through all the oxygen atoms being 0.428(3) Å. This arrangement contrasts with other recently reported 2-D water layers where the structural motifs are dominated by puckered water rings.7 The OH⋯O hydrogen bond distances within the water sheets of 2 range from 2.750(4) to 2.926(4) with the average value at 2.75(5) Å. This can be compared with the OH⋯O distance in ice Ih (2.759 Å at 200 K)10 and liquid water (2.85 Å).11 Average OH⋯O distances reported for other 2-D water layers include 2.748,7b 2.847d and 2.85 Å.1c
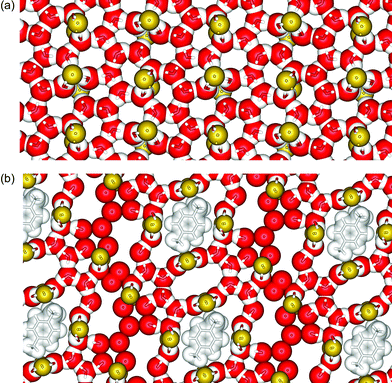 | ||
| Fig. 3 (a) A section of one of the 2-D water sheets in 2 and (b) a section of one of the 2-D clathrate hydrate layers in 3. The fluoride ligands from the sandwiching arrays of 1 are coloured yellow. | ||
When 1 is crystallised from toluene/cyclohexane containing trace amounts of water (mole fraction ca. 1.5 × 10−3), crystals of composition 6[CuF(tptm)]·14.5(H2O)·0.5(toluene) (3) are formed. The same crystalline phase can be obtained if 1 is crystallised from dichloromethane/cyclohexane solutions that contain traces of water and toluene. The single crystal X-ray structure determination‡ of 3 reveals that there are six independent complex molecules, 14.5 independent water molecules and 0.5 toluene molecules in the asymmetric unit.
Remarkably, the overall structure of 3 is very similar to that found for 2. The molecules of 1 stack together as before in pairs of flat sheets (maximum deviation of the copper atoms from the mean plane through all the copper atoms in any one plane is less than 0.0523(3) Å) with the fluoride ligands all directed inwards towards a sandwiched, 2-D mono-layer comprised of both water and toluene. As in the structure of 2, the three dimensional crystal lattice can be viewed as being built up of stacks of these composite sheets, and this is clearly illustrated in Fig. 1b.
The major difference between these two structures involves the 2-D mono-layer of water and toluene, and the way in which the two sandwiching layers interact with it. Unlike the situation in 2, where the molecules of 1 in the two sandwiching arrays are out of register, in 3 the molecules of 1 in the corresponding pairs of arrays are almost perfectly eclipsed (see diagrammatic representation in Fig. 2). Each pair of fluoride ligands is bridged by two water molecules from the water layer with OH⋯F distances of 2.594(5) and 3.080(7) Å. In this eclipsed arrangement the inter-layer separation is expanded to 4.04(1) Å from the corresponding value of 3.92(4) Å that is found in 2.
The other water molecules within the water sheets of 3 form hydrogen bonds to these bridging water molecules and also with each other to form a relatively open hydrogen bonded network that consists of 4-, 5-, 8-, 10-, 14- and 16-membered rings, each of which contains one or more fluoride bridges (see Fig. 3b). The water sheets are even more planar than in 2 (maximum deviation of oxygen atoms from the mean plane through all the oxygen atoms is 0.301(4) Å), even though the inter-layer separation is larger in this case (4.04(1) vs. 3.92(4) Å for 2). The O⋯O hydrogen bond distances within the water sheets range from 2.58(2) to 2.99(2) with the average value being 2.78(9) Å. This is very close to the average value found for the corresponding distances in 2.
The toluene molecules lie at regular intervals within the water sheets with the planes of the aromatic rings parallel to the sheets. Each toluene molecule occupies a cavity that is bounded by a ring of 16 water molecules, 8 of which are involved in forming bridges to fluorides. The size and shape of the cavity are such that the enclosed toluene is found with the methyl group oriented in one of two equivalent positions due to the crystallographic inversion centre. A superposition of these two possible arrangements for the toluene molecules is shown in Fig. 3b. The closest approaches to toluene made by the atoms of the encapsulating molecules fall outside van der Waals contact distances. The hydrogen atoms of the surrounding water molecules are all involved in hydrogen bonds that are directed either parallel to, or away from the outer surface of the toluene molecule, consistent with currently accepted hydrophobic hydration models.2a–d In addition to toluene, it is possible to incorporate other aromatic molecules such as benzene, o-, m- and p-xylene, but not naphthalene.
In conclusion, the amphiphilic molecule 1 has a number of special properties that facilitates its assembly into supramolecular bilayers that encapsulate a 2-D water sheet during crystallisation. These include its ability to form both strong OH⋯F hydrogen bonds to water and efficient intermolecular π–π stacking interactions between mercaptopyridyl groups. Remarkably, the same overall composite structure that is observed for 2 is retained when toluene is incorporated in the water sheets. Through appropriate adjustment of the register between the two sandwiching metal complex layers, strong hydrogen bonds between pairs of fluorides and water molecules are maintained while the inter-layer separation is increased and cavities of the appropriate size to encapsulate toluene are opened up within the water layer. These structural studies provide information relevant to the behaviour of water in closely confined 2-D environments, and provide the first example of a crystalline 2-D clathrate hydrate.
Notes and references
- (a) R. Ludwig, Angew. Chem., Int. Ed., 2001, 40, 1808–1827 CrossRef CAS; (b) I. Ohmine and S. Saito, Acc. Chem. Res., 1999, 32, 741–749 CrossRef CAS; (c) K. Raghuraman, K. K. Katti, L. J. Barbour, N. Pillarsetty, C. L. Barnes and K. V. Katti, J. Am. Chem. Soc., 2003, 125, 6955–6961 CrossRef CAS.
- (a) V. V. Yaminsky and E. A. Vogler, Curr. Opin. Colloid Interface Sci., 2001, 6, 342–349 Search PubMed; (b) S. Otto and J. B. F. N. Engberts, Org. Biomol. Chem., 2003, 1, 2809–2820 RSC; (c) G. Graziano, J. Chem. Soc., Faraday Trans., 1998, 94, 3345–3352 RSC; (d) L. R. Pratt and A. Pohorille, Chem. Rev., 2002, 102, 2671–2692 CrossRef CAS; (e) W. Blokzijl and J. B. F. N. Engberts, Angew. Chem., Int. Ed. Engl., 1993, 32, 1545–1579 CrossRef; (f) K. A. Udachin and J. A. Ripmeester, Nature, 1999, 397, 420–422 CrossRef CAS.
- (a) A. I. Kolesnikov, J.-M. Zanotti, C.-K. Loong, P. Thiyagarajan, A. P. Moravsky, R. O. Loutfy and C. J. Burnham, Phys. Rev. Lett., 2004, 93, 35503 1–4; (b) J. W. Lynn, Q. Huang, C. M. Brown, V. L. Miller, M. L. Foo, R. E. Schaak, C. Y. Jones, E. A. Mackey and R. J. Cava, Phys. Rev. B, 2003, 68, 214516 1–7 Search PubMed.
- R. Bergman and J. Swenson, Nature, 2000, 403, 283–286 CrossRef CAS.
- (a) L. J. Barbour, G. W. Orr and J. L. Atwood, Nature, 1998, 393, 671–673 CrossRef; (b) W. B. Blanton, S. W. Gordon–Wylie, G. R. Clark, K. D. Jordon, J. T. Wood, U. Geiser and T. J. Collins, J. Am. Chem. Soc., 1999, 121, 3551–3552 CrossRef CAS.
- R. Custelcean, C. Afloroaei, M. Vlassa and M. Polverejan, Angew.Chem., Int. Ed., 2000, 39, 3094–3096 CrossRef CAS.
- (a) P. Rodríguez-Cuamatzi, G. Vargas-Díaz and H. Höpfl, Angew. Chem., Int. Ed., 2004, 43, 3041–3044 CrossRef CAS; (b) B.-Q. Ma, H.-L. Sun and S. Gao, Angew. Chem., Int. Ed., 2004, 43, 1374–1376 CrossRef CAS; (c) C. Janiak, T. G. Scharmann and S. A. Mason, J. Am. Chem. Soc., 2002, 124, 14010–14011 CrossRef CAS; (d) K.-M. Park, R. Kuroda and T. Iwamoto, Angew. Chem., Int. Ed., 1993, 32, 884–886 CrossRef.
- I. Kinoshita, L. J. Wright, S. Kubo, K. Kimura, A. Sakata, T. Yano, R. Miyamoto, T. Nishioka and K. Isobe, Dalton Trans., 2003, 1993–2003 RSC.
- M. Scudder and I. Dance, Chem. Eur. J., 2002, 8, 5456–5468 CrossRef CAS.
- D. Eisenberg and W. Kauzmann, The structure and properties of water, Oxford University Press, London, 1969, p. 74 Search PubMed.
- W. F. Kuhs and M. S. Lehmann, J. Phys. Chem., 1983, 87, 4312–4313 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: experimental descriptions. See http://www.rsc.org/suppdata/cc/b5/b505681k/index.sht |
‡ Crystal data for 2: C64H72Cu4F4N12O12S12; Mr
= 1916.24, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (#2), a
= 14.220(2)
Å, b
= 16.120(2)
Å, c
= 18.390(2)
Å, α
= 73.370(5)°, β
= 89.560(7)°, γ
= 84.760(6)°, V
= 4021.5(8)
Å3, T
= 193(1) K, μ(Mo-Kα)
= 1.428 mm−1, 38862 reflections were measured, 17899 independent reflections with 12253 having I > 2σ(I), 1081 parameters, R1(I > 2σ(I))
= 0.0530, wR2
(all data)
= 0.1230, GOF = 1.011. Crystal data for 3: C99.5H105Cu6F6N18O14.5S18; Mr
= 2857.38, triclinic, P
(#2), a
= 14.220(2)
Å, b
= 16.120(2)
Å, c
= 18.390(2)
Å, α
= 73.370(5)°, β
= 89.560(7)°, γ
= 84.760(6)°, V
= 4021.5(8)
Å3, T
= 193(1) K, μ(Mo-Kα)
= 1.428 mm−1, 38862 reflections were measured, 17899 independent reflections with 12253 having I > 2σ(I), 1081 parameters, R1(I > 2σ(I))
= 0.0530, wR2
(all data)
= 0.1230, GOF = 1.011. Crystal data for 3: C99.5H105Cu6F6N18O14.5S18; Mr
= 2857.38, triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (#2), a
= 16.650(3)
Å, b
= 16.850(3)
Å, c
= 23.630(3)
Å, α
= 78.230(6)°, β
= 67.420(5)°, γ
= 84.610(6)°, V
= 6004.4(17)
Å3, T
= 193(1) K, μ(Mo-Kα)
= 1.432 mm−1, 48292 reflections were measured, 27082 independent reflections with 21106 having I > 2σ(I), 1566 parameters, R1(I > 2σ(I))
= 0.0580, wR2
(all data)
= 0.1760, GOF = 1.008. CCDC 260665 and 260666. See http://www.rsc.org/suppdata/cc/b5/b505681k/index.sht for crystallographic data in CIF or other electronic format.
(#2), a
= 16.650(3)
Å, b
= 16.850(3)
Å, c
= 23.630(3)
Å, α
= 78.230(6)°, β
= 67.420(5)°, γ
= 84.610(6)°, V
= 6004.4(17)
Å3, T
= 193(1) K, μ(Mo-Kα)
= 1.432 mm−1, 48292 reflections were measured, 27082 independent reflections with 21106 having I > 2σ(I), 1566 parameters, R1(I > 2σ(I))
= 0.0580, wR2
(all data)
= 0.1760, GOF = 1.008. CCDC 260665 and 260666. See http://www.rsc.org/suppdata/cc/b5/b505681k/index.sht for crystallographic data in CIF or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |
