A new self-assembling capsule via metal coordination†
Takeharu
Haino
*,
Mutsumi
Kobayashi
,
Midori
Chikaraishi
and
Yoshimasa
Fukazawa
*
Department of Chemistry, Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima, 739-8526, Japan. E-mail: haino@sci.hiroshima-u.ac.jp; Fax: +81-82-424-0724; Tel: +81-82-424-7403
First published on 7th April 2005
Abstract
A new octadentate cavitand forms a stable dimeric molecular capsule via metal-coordination, creating a large and elaborate three-dimensional cavity in which large aromatic guests are accommodated to form supramolecular complexes.
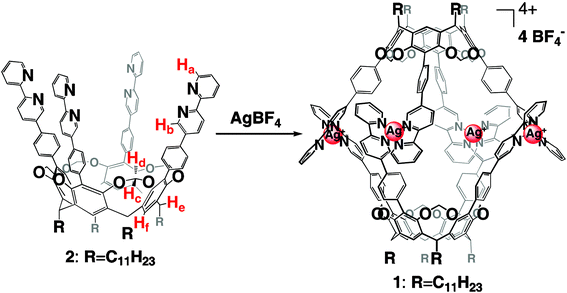
There is considerable interest in constructing nano size supramolecular hosts via self-assembly of molecular precursors.1 Such large and elaborate molecular architectures offer well-defined inner cavities capable of carrying fascinating functions: encapsulation of a large molecular guest or several guests,2 stabilizing unstable intermediates,3,4 as molecular catalysts5,6etc. These properties are unique to the assembled state, and depend on the size and nature of the cavities. Thus the development of a new class of nano size capsules via self-assembly offers the possibility to create novel functions. The resorcinarene-based cavitands are potential building blocks for creating self-assembling molecular capsules driven by hydrogen bonding1–3,5 and metal-coordination.7 We report here a new class of the metal-assisted self-assembling nano capsule 1·4BF4 using octadentate cavitand 2 and its unique guest binding properties (Scheme 1).
 | ||
| Scheme 1 Metal-induced capsule formation. | ||
The synthesis of 2 was achieved according to Scheme 2. Bipyridine unit 5 was prepared by Suzuki coupling of bromobipyridine 38 and 4.9 Cavitand 610 was treated with n-BuLi, subsequent addition of iodine to give tetra-iodocavitand 7. Suzuki coupling reaction11 of 5 and 7 smoothly proceeded to give the desired octadentate cavitand 2.
 | ||
| Scheme 2 Reagents and conditions. a) Pd(PPh3)4, Na2CO3, dioxane, H2O 49%; b) n-BuLi, I2, THF 79%; c) PdCl2(PPh3)2, Ph3As, Cs2CO3, 5, dioxane 80%. | ||
Capsule 1·4BF4 is formed instantly as a colorless solid by the simple addition of two equivalents of silver tetrafluoroborate to a solution of 2 in nitromethane. The structure of 1·4BF4 was confirmed by elementary analysis, and ESI-MS.
Many diastereomeric forms are possible for dimeric structure 1·4BF4; however, the 1H NMR spectrum in CDCl3(Fig. 1) revealed the selective formation of the D4 symmetric form in which the eight bipyridyl groups are magnetically equivalent. Pyridyl protons Ha and Hb showed upfield shifts as a consequence of the Ag–N bond formation. This rationalizes that a silver cation complexes to two bipyridines in a tetrahedral fashion; protons Ha and Hb stay in the shielding region of the other bipyridyl group and experience their shielding effect. While the precise reason for the selective formation of the D4 form remains to be determined, the molecular modeling of 1·4BF4 suggested that the D4 symmetric form is more stable than the others.12
 | ||
| Fig. 1 1H NMR spectra (500 MHz) in chloroform-d at 298 K. a) 2 (1.0 mM); b) 1·4BF4 (0.4 mM) and 2 (1.0 mM); c) 1·4BF4 (1.0 mM). | ||
Diffusion coefficients D (3.66 ± 0.05 × 10−10 and 4.79 ± 0.04 × 10−10 m2 s−1 for 1·4BF4 and 2) in chloroform were measured by pulsed field gradient NMR experiments using BPPSTE pulse sequence. The resulting ratio Ddim/Dmono = 0.76 is in reasonable agreement with the theoretical ratio of 0.72–0.75 expected for a dimer13 and confirms the dimeric nature of the complex 1·4BF4.
The complex shows high kinetic stability in organic solution. When a chloroform-d solution of 1·4BF4 was added to that of 2, their sharp signals appeared independently; the traits support sizable energetic barriers between free and complexed states. 2D ROESY spectra of the mixture revealed that the dynamic exchange between them still exists, but the rate is slower than NMR time scale. In common Ag-bipyridine complexes the energetic barrier for the exchange between the free and the complexed states are small.14 This high kinetic stability of 1·4BF4 would be a result of the cooperative complexation of the bipyridine units.
Rigid guests 8–13 allow us to measure the experimental interior dimension of the capsule (Fig. 2). 4,4′-Diacetoxybiphenyl 9 is readily encapsulated by 1 in CDCl3, and the complex formed shows high kinetic and thermodynamic stability (Fig. 3a, Table 1). The large upfield shift of the guest methyl groups (δ: −1.37, Δδ: −3.70 ppm) was observed. 2D NOESY spectrum showed strong intermolecular NOE cross-peaks between the methyl protons of the bound guest and the Hc, Hd and Hf protons on the resorcinarene platform. The large upfield shift and these NOE's are consistent with the binding conformation in which the two methyl groups are close to each end of the cavity. The complex of 10 also showed the high kinetic stability with the large upfield shift of the methyl group (δ: 0.4, Δδ: −3.5 ppm) (Fig. 3b), but the association constant is about 200 times as small as that of 9 even though they have similar and complementary shapes (molecular lengths: 14.2 and 14.1 Å for 9 and 10). It is known that the resorcinarene cavity creates CH/π interaction to acidic protons,15 so that the more acidic methyl proton of 9 should drive the extremely high binding ability. In contrast, 8 did not complex whereas the other aromatic guests 11, 12 and 13 were encapsulated, but the time-averaged spectra were given. Accordingly, 9 and 10 establish the best complementary molecular length, about 14 Å.
 | ||
| Fig. 2 Guests. | ||
 | ||
| Fig. 3 The upfield region of the 1H NMR spectra in chloroform-d of 1·4BF4 (2.0∼3.6 mM) with: a) 9 (1.5 eq.) at 298 K, b) 10 (1.0 eq.) at 298 K, c) the 1∶1 mixture of 14 (3.5 eq.) and acetic acid (3.5 eq.) at 218 K, d) the 1∶1 mixture of 14 (8.0 eq.) and propionic acid (8.0 eq.) at 218 K. | ||
| Guest | K a | Guest | K a |
|---|---|---|---|
| a Association constants were determined at 298 K. | |||
| 8 | 0 | 11 | 80 ± 10 |
| 9 | 82000 ± 6000 | 12 | 118 ± 5 |
| 10 | 410 ± 10 | 13 | 60 ± 8 |
Two different carboxylic acids form the statistical mixtures of the homo and the hetero hydrogen-bonded dimers in organic solution; however, the capsule enhanced the hetero dimerization of carboxylic acids.16 When a 1∶1 mixture of acetic acid and 14 was added to the solution of 1·4BF4, an asymmetrically filled capsule was exclusively observed at 218 K (Fig. 3c). Two singlets appeared in the clear window higher than 0 ppm. Based on 2D ROESY experiment, they are assigned to the methyl groups of 14 and acetic acid (δ: −1.43; Δδ: −3.8 ppm for AcO–, δ: −1.54; Δδ: −3.7 ppm for acetic acid). These characteristic upfield shifts of the methyl groups and the integrations confirmed the formation of the hetero dimer of 14 and acetic acid in the capsule. Propionic acid also forms the hetero dimer with 14 in the capsule. The characteristic upfield shifts were observed for –CH3 and –CH2– of propionic acid (Δδ: −3.1 and Δδ: −2.0 ppm for –CH3 and –CH2–), placing both –CH3 groups near the ends of the cavity (Fig. 3d).
The relative stability of the hetero dimers was assessed through the competition experiment. When acetic acid, propionic acid and 14 were added in a 1∶1∶1 ratio to a chloroform solution of 1·4BF4, the hetero dimer of 14 and acetic acid formed exclusively. The calculated structures suggest that both hetero dimers fit nicely inside and the methyl groups of acetic and propionic acids point down to the end of the cavity, creating CH/π interactions (Fig. 4).12 The more acidic methyl group of acetic acid receives more attractive CH/π interaction to the aromatic cavity than that of propionic acid. Accordingly, the acidity of the methyl groups most likely drives the selectivity.
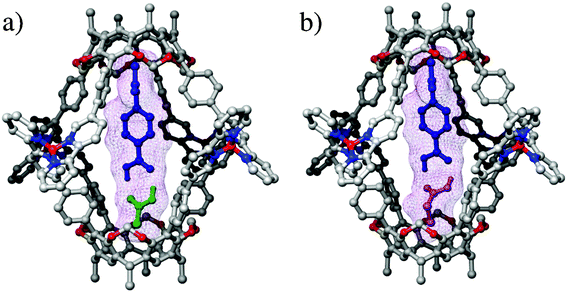 | ||
| Fig. 4 Optimized structures of co-encapsulation complexes of 1 with 14 and: a) acetic acid; b) propionic acid. | ||
In summary, we demonstrated the synthesis of self-assembled capsule 1·4BF4 and its unique encapsulation properties, derived from its size and shape. Matching the overall length of the H-bonded pair of two different carboxylic acids with the dimension of the cavity results in the uncommon co-encapsulation with surprising selectivity. Further studies on the binding properties of 1·4BF4, as well as other related self-assembling capsules, are currently underway.
Notes and references
- (a) F. Hof, S. L. Craig, C. Nuckolls and J. Rebek, Jr., Angew. Chem., Int. Ed., 2002, 41, 1488–1508 CrossRef CAS; (b) L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem., Int. Ed., 2001, 40, 2382–2426 CrossRef CAS; (c) J. Rebek, Jr., Acc. Chem. Res., 1999, 32, 278–286 CrossRef.
- (a) T. Heinz, D. M. Rudkevich and J. Rebek, Jr., Nature, 1998, 394, 764–766 CrossRef CAS; (b) M. Yamanaka and J. Rebek, Jr., Chem. Commun., 2004, 1690–1691 RSC; (c) A. Shivanyuk and J. Rebek, Jr., J. Am. Chem. Soc., 2002, 124, 12074–12075 CrossRef CAS; (d) T. Amaya and J. Rebek, Jr., Chem. Commun., 2004, 1802–1803 RSC.
- S. K. Körner, F. C. Tucci, D. M. Rudkevich, T. Heinz and J. Rebek, Jr., Chem.–Eur. J., 2000, 6, 187–195 CrossRef CAS.
- T. Kusukawa and M. Fujita, J. Am. Chem. Soc., 1999, 121, 1397–1398 CrossRef CAS.
- (a) B. W. Purse, P. Ballester and J. Rebek, Jr., J. Am. Chem. Soc., 2003, 125, 14682–14683 CrossRef CAS; (b) J. Kang and J. Rebek, Jr., Nature, 1997, 385, 50–52 CrossRef CAS.
- (a) M. Yoshizawa, S. Miyagi, M. Kawano, K. Ishiguro and M. Fujita, J. Am. Chem. Soc., 2004, 126, 9172–9173 CrossRef CAS; (b) M. Yoshizawa, Y. Takeyama, T. Kusukawa and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 1347–1349 CrossRef CAS.
- (a) P. Jacopozzi and E. Dalcanale, Angew. Chem., Int. Ed. Engl., 1997, 36, 613–615 CrossRef CAS; (b) B. Bibal, B. Tinant, J.-P. Declercq and J.-P. Dutasta, Chem. Commun., 2002, 432–433 RSC; (c) S. J. Park and J.-I. Hong, Chem. Commun., 2001, 1554–1555 RSC; (d) K. Kobayashi, Y. Yamada, M. Yamanaka, Y. Sei and K. Yamaguchi, J. Am. Chem. Soc., 2004, 126, 13896–13897 CrossRef CAS.
- F. M. Romero and R. Ziessel, Tetrahedron Lett., 1995, 36, 6471–6474 CrossRef CAS.
- M. H. Todd, S. Balasubramanian and C. Abell, Tetrahedron Lett., 1997, 38, 6781–6784 CrossRef CAS.
- P. Timmerman, H. Boerrigter, W. Verboom, G. J. Van Hummel, S. Harkema and D. N. Reinhoudt, J. Inclusion. Phenom. Mol. Recognit. Chem., 1994, 19, 167–191 CrossRef.
- L. Sebo, F. Diederich and V. Gramlich, Helv. Chim. Acta, 2000, 83, 93–113 CrossRef CAS.
- The molecular mechamics calcuations were carried out by MacroModel V. 6.5 using amber* force field. F. Mohamadi, N. G. J. Richards, W. C. Guida, R. Liskamp, M. Lipton, C. Caufield, G. Chang, T. Hendrickson and W. C. Still, J. Comp. Chem., 1990, 11, 440 Search PubMed.
- (a) M. S. Kaucher, Y.-F. Lam, S. Pieraccini, G. Gottarelli and J. T. Davis, Chem.–Eur. J., 2005, 11, 164–173 CrossRef; (b) F. W. Kotch, V. Sidorov, Y.-F. Lam, K. J. Kayser, H. Li, M. S. Kaucher and J. T. Davis, J. Am. Chem. Soc., 2003, 125, 15140–15150 CrossRef CAS; (c) B. Olenyuk, M. D. Levin, J. A. Whiteford, J. E. Shield and P. J. Stang, J. Am. Chem. Soc., 1999, 121, 10434–10435 CrossRef CAS; (d) A. S. Altieri, D. P. Hinton and R. A. Byrd, J. Am. Chem. Soc., 1995, 117, 7566–7567 CrossRef CAS; (e) V. V. Krishnan, J. Magn. Reson., 1997, 124, 468–473 CrossRef CAS.
- T. Haino, H. Araki, Y. Yamanaka and Y. Fukazawa, Tetrahedron Lett., 2001, 42, 3203–3206 CrossRef CAS.
- (a) K. Kobayashi, Y. Asakawa, Y. Kikuchi, H. Toi and Y. Aoyama, J. Am. Chem. Soc., 1993, 115, 2648–2654 CrossRef CAS; (b) D. Falábu, A. Shivanyuk, M. Nissinen and K. Rissanen, Org. Lett., 2002, 4, 3019–3022 CrossRef CAS.
- Benzoic acids and methylpyridone formed homo dimers in the self-assembled cylindrical capsule. T. Heinz, D. M. Rudkevich and J. Rebek, Jr., Angew. Chem., Int. Ed., 1999, 38, 1136–1139 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: experimental section, ESI MS of the capsule, and 2D spectra of the complexes. See http://www.rsc.org/suppdata/cc/b5/b502598b/ |
| This journal is © The Royal Society of Chemistry 2005 |
