A supramolecular approach to multivalent target-specific MRI contrast agents for angiogenesis†
Anouk Dirksena, Sander Langereisa, Bas F. M. de Waala, Marcel H. P. van Genderena, Tilman M. Hackeng*b and E. W. Meijer*a
aLaboratory of Macromolecular and Organic Chemistry, Eindhoven University of Technology, P. O. Box 513, 5600 MB, Eindhoven, The Netherlands. E-mail: E.W.Meijer@tue.nl; Fax: +31 (0)40 2451036; Tel: +31 (0)40 2473101
bCardiovascular Research Institute Maastricht (CARIM), University Maastricht, P. O. Box 616, 6200 MD, Maastricht, The Netherlands. E-mail: T.Hackeng@bioch.unimaas.nl; Fax: +31 (0)43 3884159; Tel: +31 (0)43 3881538
First published on 14th April 2005
Abstract
The synthesis of a cyclic peptide–Gd(III)DTPA molecule equipped with biotin is presented, yielding a well-defined multivalent MRI contrast agent after its coupling to avidin.
Magnetic Resonance Imaging (MRI) is a powerful, non-invasive technique which plays an important role in clinical diagnosis. To enhance the contrast in T1-weighted MR images, gadolinium(III) (Gd(III)) chelates are commonly used as MRI contrast agents.1 Despite the efforts to improve contrast in MR images, some processes of interest, such as angiogenesis (the formation of new blood vessels), are difficult to visualize with MRI. Target-specific MRI contrast agents2 designed to bind to proteins expressed by the cells involved in angiogenesis could improve the imaging of this process drastically due to the in situ accumulation of MRI contrast agent. A cyclic peptide containing the asparagine–glycine–arginine (NGR) sequence (cNGR) was identified as a specific ligand for the aminopeptidase CD13, a protein over-expressed by angiogenic endothelial cells.3,4 By immobilizing multiple cNGR moieties and MRI labels on one single carrier, the binding of cNGR to CD13 may be improved through cooperative binding (multivalency),5 resulting in a stronger accumulation of MRI contrast agent around regions of angiogenesis. Next to targeting, sensitivity is the other major challenge for molecular MR imaging. One way to increase the contrast is the connection of multiple Gd(III) chelates to one carrier.1,2 High local concentrations of Gd(III) can be reached, while due to a higher molecular weight the T1 of water molecules is more effectively reduced as compared to their monovalent low molecular weight analogues.
A strategy to synthesize such a multivalent target-specific MRI contrast agent comprises the self-assembly of monovalent target-specific MRI contrast agent units onto the periphery of a large carrier with high selectivity and high affinity. Avidin, a tetrameric protein, is capable of binding four equivalents of biotin in a strong, non cooperative fashion (Kass ≈ 1.7 × 1015 M−1)6 and has been successfully employed as a versatile supramolecular scaffold for the synthesis of large well-defined structures.7 In this paper we describe the synthesis of a well-defined multivalent target-specific MRI contrast agent based on the biotin–avidin system. For this, cNGR was functionalized with both the MRI label gadolinium(III) diethylene triaminepentaacetic acid (Gd(III)DTPA) and biotin (7) (Scheme 1).
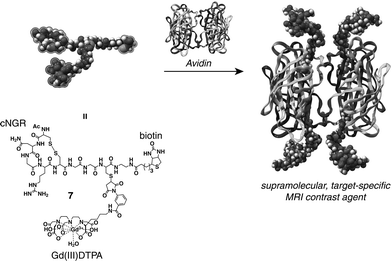 | ||
| Scheme 1 Supramolecular approach for the synthesis of a multivalent target-specific contrast agent based on 7 and avidin. | ||
The synthesis of 7 comprises a sequence of highly efficient, chemoselective reaction steps, which we recently introduced as a general strategy for the double labeling of peptides.8 Biotinylated cysteine (2) was reacted with the C-terminal thioester of peptide 1 containing the target-specific NGR sequence under native chemical ligation conditions (Scheme 2). This methodology was previously described by Dawson et al. for the synthesis of proteins of moderate size.9,10 At this point both cysteine residues of the peptide are protected with acetamidomethyl (Acm) groups to ensure a correct “folding” of the peptide later on. The reaction was monitored employing analytical reversed phase HPLC (RP HPLC) using a C18 column for separation coupled to UV-Vis (λprobe = 214 nm). Within 2 hours the thioester-functionalized peptide 1 reacted quantitatively with 2. The ligation product was purified using preparative RP HPLC on a C18 column and subsequent lyophilization rendered 3 in 83% yield. Subsequently, the sulfhydryl group of the cysteine residue that was involved in the ligation reaction was utilized to introduce the DTPA ligand through the reaction of 3 with maleimide-functionalized DTPA (4) (Scheme 2). The reaction was monitored employing analytical RP HPLC coupled to UV-Vis (λprobe = 214 nm). Once the reaction went to completion the reaction mixture was diluted ∼30 times with 0.1 M Tris (aq, pH 6.5) after which 10 vol% of acetic acid was added. Then 1.75 equivalents of I2 were added to remove the Acm protecting groups of 5. The removal of the Acm groups resulted instantaneously in the correct “folding” of the peptide unit into its cyclic conformation 6 (Scheme 2). Purification using preparative RP HPLC on a C18 column and subsequent lyophilization gave 6 in 27% yield. The corresponding Gd(III)-complex 7 was obtained in quantitative yield (> 99%) through the addition of 1 equivalent of GdCl3 in H2O (Scheme 2). The formation of 7 was confirmed with ESI-MS.
 | ||
| Scheme 2 The synthesis of the biotinylated target-specific contrast agent 7. (i) 2 vol% thiophenol, 2 vol% benzylmercaptan, 6 M guanidine in 0.1 M Tris, 1 h, pH ≈ 7, 37 °C; (ii) spontaneous rearrangement; (iii) 0.1 M Tris, 1 h, pH 6.5, RT; (iv) 1.75 equiv. I2, 10 vol% acetic acid, 1 h, RT; (v) 1 equiv. GdCl3, H2O, pH 6.5–7; (Acm = –CH2CONHCH3). | ||
To verify the binding stoichiometry between 7 and avidin, the HABA (4′-hydroxyazobenzene-2-carboxylic acid) assay was performed probing the UV-Vis absorption at 500 nm.11 Upon addition of 7 to a solution of avidin in PBS buffer containing 5 equivalents of HABA per binding site, HABA is expelled from the biotin binding pocket of avidin, resulting in a decrease in the absorption at 500 nm of HABA bound to avidin (Fig. 1). After the addition of 3.8 equivalents of 7 the absorption spectrum at 500 nm does not change any further, indicating that all binding sites of avidin are occupied by 7.
 | ||
| Fig. 1 HABA assay showing the decrease in UV-Vis absorption at 500 nm upon the addition of 7 (0.26 mM in PBS buffer, pH 7.4) to a 0.5 mL solution of avidin (10 µM in PBS buffer, pH 7.4) containing 5 equiv. of HABA per binding site (the dashed lines through the data points serve to guide the eye). | ||
The longitudinal relaxivity r1, which is an intrinsic property of the MRI contrast agent, was determined from concentration dependent measurements of the longitudinal relaxation time (T1) of 7 at 1.5 T at 20 °C. The data gave a good linear fit to the equation (1/T1)observed = (1/T1)diamagnetic + r1[Gd(III)] (R2 > 0.999) and an r1 of 8.1 mM−1s−1 was calculated. This value is higher than the r1 of parent Gd(III)DTPA (r1 = 4.2 mM−1s−1 at 1.5 T and 20 °C), which can be explained in terms of molecular weight (1.9 kDa for 7versus 0.5 kDa for Gd(III)DTPA).
To gain insight into the effect of binding of 7 to avidin on the r1 of 7 an E-titration12 was performed using MR (at 1.5 T and 20 °C), adding a 0.12 mM solution of avidin in PBS buffer (pH 7.4) to a 0.08 mM solution of 7 in PBS buffer (pH 7.4). This resulted in a linear increase in the r1 of 7 (Fig. 2). The addition of more than 0.24 equivalents of avidin did not result in any further changes in the r1 of 7 (Fig. 2). This behavior rules out non-specific binding between 7 and avidin.
 | ||
| Fig. 2 E-titration12 at 1.5 T and 20 °C showing the r1 of 7 (0.08 mM in PBS buffer, pH 7.4) in the presence of increasing amounts of avidin (dotted line: fitted data with r1,free = 8.1 mM−1s−1, yielding r1,bound = 13.8 ± 0.5 mM−1s−1 and N = 4.1 ± 0.3). | ||
In a previous study we found that the r1 of biotinylated Gd(III)DTPA increases by a factor of 3 from r1,free = 6.1 mM−1s−1 to r1,bound = 17.5 mM−1s−1 upon binding to avidin.13 This result was assigned to a strong reduction in the molecular tumbling rate of the Gd(III)DTPA moiety upon binding of the biotinylated Gd(III)DTPA contrast agent to the large protein avidin (molecular weight of 64 kDa). By fitting the data of the E-titration to a mathematical model describing the binding of multiple substrates to a multivalent protein with N identical, independent binding sites,13 and by taking into account that Kass = 1.7 × 1015 M−1 and r1,free = 8.1 mM−1s−1, an r1,bound of 13.8 ± 0.5 mM−1s−1 and an N of 4.1 ± 0.3 were calculated. Remarkably, the increase in r1 of 7 by a factor of 1.7 is not as pronounced as in the case of biotinylated Gd(III)DTPA, where we found a 3-fold increase. This may be attributed to the longer spacer between the Gd(III)DTPA moiety and the biotin unit in the case of 7, which maintains a higher degree of flexibility for the Gd(III)DTPA moiety.
In conclusion, by exploiting the strong and specific binding of biotin to avidin, a well-defined multivalent target-specific MRI contrast agent based on avidin and 7 was synthesized through self-assembly. The availability of nanoparticles coated with avidin14 allows an even higher loading of Gd(III)DTPA and cNGR peptide units per carrier. The profits of a high loading of Gd(III)DTPA moieties as well as target-specific peptides will be investigated in vivo using a murine model to assess its efficacy as a multivalent target-specific MRI contrast agent.
We kindly acknowledge W. Adriaens for her support during peptide synthesis and K. Pieterse for the artwork. This work was financially supported by the Council for Chemical Sciences of the Netherlands Organization for Scientific Research and by the BSIK-program entitled ‘Molecular Imaging of Ischemic Heart Disease’ (project number BSIK03033).
Notes and references
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS.
- V. Jacques and J. F. Desreux, Top. Curr. Chem., 2002, 221, 123–164 CAS.
- F. Curnis, G. Arrigoni, A. Sacchi, L. Fischetti, W. Arap, R. Pasqualini and A. Corti, Cancer Res., 2002, 62, 867–874 CAS.
- R. Pasqualini, E. Koivunen and E. Ruoslahti, Nat. Biotechnol., 1997, 15, 542–546 CrossRef CAS.
- For example: M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754–2794 Search PubMed.
- N. M. Green, Methods Enzymol., 1990, 184, 51–67 CAS.
- For example: (a) W. Müller, H. Ringsdorf, E. Rump, G. Wildburg, X. Zhang, L. Angermaier, W. Knoll, M. Liley and J. Spinke, Science, 1993, 262, 1706–1708 CrossRef CAS; (b) H. Ringsdorf and J. Simon, Nature, 1994, 371, 284 CrossRef CAS; (c) C. M. Niemeyer, M. Adler, B. Pignataro, S. Lenhert, S. Gao, L. Chi, H. Fuchs and D. Blohm, Nucleic Acids Res., 1999, 27, 4553–4561 CrossRef CAS; (d) C. M. Niemeyer, W. Burger and J. Peplies, Angew. Chem., Int. Ed., 1998, 37, 2265–2268 CrossRef CAS; (e) S. A. Anderson, R. K. Rader, W. F. Westlin, C. Null, D. Jackson, G. M. Lanza, S. A. Wickline and J. J. Kotyk, Magn. Reson. Med., 2000, 44, 433–439 CrossRef CAS; (f) H. Kobayashi, S. Kawamoto, R. A. Star, T. A. Waldmann, M. W. Brechbiel and P. L. Choyke, Bioconjugate Chem., 2003, 14, 1044–1047 CrossRef CAS.
- A. Dirksen, S. Langereis, B. F. M. de Waal, M. H. P. van Genderen, E. W. Meijer, Q. G. de Lussanet and T. M. Hackeng, Org. Lett., 2004, 6, 4857–4860 CrossRef CAS.
- P. E. Dawson, T. W. Muir, I. Clark-Lewis and S. B. H. Kent, Science, 1994, 266, 776–779 CrossRef CAS.
- T. M. Hackeng, J. H. Griffin and P. E. Dawson, Proc. Natl. Acad. Sci. USA, 1999, 96, 10068–10073 CrossRef CAS.
- N. M. Green, Biochem. J., 1965, 94, 23c–24c CAS.
- Nuclear Magnetic Resonance in Biochemistry. Applications to Enzyme Systems, Clarendon Press, Oxford, 1973 Search PubMed.
- S. Langereis, H.-A. T. Kooistra, M. H. P. van Genderen and E. W. Meijer, Org. Biomol. Chem., 2004, 2, 1271–1273 RSC.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: synthesis and characterization of all compounds reported in this paper and a description of the instrumentation used. See http://www.rsc.org/suppdata/cc/b5/b502347e/ |
| This journal is © The Royal Society of Chemistry 2005 |
