Catalytic asymmetric synthesis of enantiopure isoprenoid building blocks: application in the synthesis of apple leafminer pheromones†
Ruben P.
van Summeren
,
Sven J. W.
Reijmer
,
Ben L.
Feringa
* and
Adriaan J.
Minnaard
*
Department of Organic and Molecular Inorganic Chemistry, Stratingh Institute, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: B.L.Feringa@chem.rug.nl., A.J.Minnaard@chem.rug.nl
First published on 9th February 2005
Abstract
The first catalytic asymmetric procedure capable of preparing all 4 diastereoisomers (ee > 99%, de > 98%) of a versatile saturated isoprenoid building block was developed and the value of this new method was demonstrated in its application to the concise total synthesis of two pheromones.
The saturated isoprenoid unit is a central theme in many natural products including pheromones,1 vitamins,2 marine natural products,3 and archaebacterial lipids.4 Isoprenoid based chiral building blocks (Fig. 1) therefore constitute a major synthetic challenge to the field of total synthesis. Current methods to synthesize such building blocks include chiral pool strategies,1b,5 enzymatic desymmetrization protocols3b,6 and chiral auxiliary based approaches.4b,7 However, all of these methods are inherently multistep and either incapable of delivering all diastereoisomers or use a stoichiometric amount of chiral material. As for asymmetric catalytic methods, only two examples have been reported in the literature. In 1987 Noyori employed a Ru–binap complex in the asymmetric hydrogenation of allylic alcohols to synthesize a C15 chain containing a saturated syn-isoprenoid unit as present in the vitamins E and K.8 More recently, Negishi developed an elegant Zr-catalyzed enantioselective carboalumination to prepare such compounds.9 It should be noted though, that in both cases only the syn-isoprenoid unit has been synthesized and that one end of the molecule is restricted to a saturated alkyl chain.
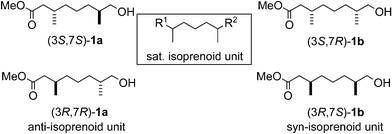 | ||
| Fig. 1 General structure of the saturated isoprenoid unit and structures of all diastereoisomers of the isoprenoid building block prepared by asymmetric catalysis. | ||
 | ||
| Scheme 1 Synthesis of enantiopure building blocks.16 (a) Cu(OTf)2 (5 mol%), L1 (10 mol%), Me2Zn (5.0 eq.), toluene, −25 °C, 12 h; (b) Cu(OTf)2 (2.5 mol%), L1 (5 mol%), Me2Zn (1.5 eq.), CH2Cl2, −25 °C, 12 h, then Et3N (3.0 eq.), TMEDA (5.0 eq.), TMSOTf (5.0 eq.), Et2Zn (0.42 eq.), rt, 2 h; (c) Cu(OTf)2 (2.5 mol%), ent-L1 (5 mol%), Me2Zn (1.5 eq.), CH2Cl2, −25 °C, 12 h, then Et3N (3.0 eq.), HMPA (5.0 eq.), TMSCl (5.0 eq.), Et2Zn (0.42 eq.), rt, 2 h; (d) O3, MeOH, CH2Cl2, −78 °C, 15 min, then NaBH4 (10 eq.), rt, 12 h; (e) MeOH, TMSCl (3.0 eq.), reflux, 12 h. | ||
 | ||
| Scheme 2 Synthesis of apple leafminer pheromones. (a) p-TsCl, pyridine, 0 °C, 12 h; (b) DIBAH (5.0 eq.), Et2O, −78 °C, 30 min; (c) CuBr·SMe2 (1.0 eq.), n-PrMgBr (16 eq.), THF, −78 °C to 0 °C, 12 h; (d) CuBr·SMe2 (21 mol%), 6-heptenylMgBr (4.0 eq.), THF, −78 °C to 0 °C, 12 h; (e) CuBr·SMe2 (31 mol%), n-hexylMgBr (5.7 eq.), THF, −78 °C to 0 °C, 12 h. | ||
In order to avoid these constraints in synthetic flexibility and stereocontrol, we have focused on a more general applicable method towards enantiopure saturated isoprenoid units. Herein we report a catalytic method which allows, for the first time, the synthesis of all 4 diastereoisomers (ee > 99%, de > 98%) of a versatile isoprenoid building block, capable of functionalization at both terminae (Fig. 1). Furthermore, we demonstrate the synthetic usefulness of the acquired building blocks by an application in the total synthesis of two pheromones.
Our method is based on the Cu-phosphoramidite catalyzed asymmetric conjugate addition of dialkylzinc reagents to cyclic substrates, developed in our group.10 We anticipated that this protocol, used iteratively in the conjugate addition of Me2Zn to cycloocta-2,7-dienone (2) followed by oxidative ring opening (Fig. 2), would enable the rapid assembly of enantiopure syn- and anti-isoprenoid building blocks. However, as depicted in Fig. 2, quenching of the zinc enolate after the second conjugate addition with a proton source would result in a meso compound in case of the cis-adduct. To avoid a racemic product after ring opening, a procedure was developed, involving in situ trapping of the zinc enolate as a silyl enol ether and subsequent ring opening via ozonolysis (Scheme 1).11
 | ||
| Fig. 2 General strategy for synthesizing enantiopure saturated isoprenoid building blocks; problem of formation of a meso compound. | ||
Cycloocta-2,7-dienone (2) was prepared from cyclooctanone in one step via oxidation with IBX as described by Nicolaou et al.12 Conjugate addition of Me2Zn to 2 yielded 3 (ee > 99%) in 85% yield using 5 mol% catalyst, 5.0 eq. Me2Zn and slow addition of the substrate to the reaction mixture over 5 h. When standard conditions were employed (2.5 mol% catalyst, 1.5 eq. Me2Zn),10c a significant amount of side product (4) was formed due to Michael addition of the zinc-enolate to the substrate (Scheme 1). In the second conjugate addition this side reaction was sufficiently suppressed by slow addition of the substrate allowing for the use of 2.5 mol% of catalyst. In the case of the trans-adduct, quenching the reaction with TMSOTf in the presence of Et3N and TMEDA resulted in quantitative formation of the corresponding silyl enol ether 5a (ee > 99%, de > 98%). The cis-adduct 5b was formed (95% conversion as determined by 1H–NMR) with excellent selectivity (ee > 99%, de > 98%) when HMPA and TMSCl were used instead of TMEDA and TMSOTf, as the latter reagents induced partial racemization.13 The silyl enol ethers (5a/5b) were ring-opened by ozonolysis and the resulting aldehyde was reduced upon work-up with NaBH4. The crude carboxylic acids (6a/6b) were then heated under reflux in MeOH in the presence of TMSCl to give the methyl esters 1a and 1b in an overall yield of 45% from 3.14 As an alternative, the conversion of 3 into carboxylic acid 6a was performed as a one pot procedure, yielding 1a after esterification in 40% yield.15 The enantiomers of all compounds in Scheme 1 were prepared by using the opposite enantiomer of the ligand, i.e. ent-L1 in place of L1 and vice versa.16
In summary, all 4 diastereoisomers of 1 were synthesized in excellent ee (>99%) and de (>98%) from 2 in 4 steps with an overall yield of 38%. It should be emphasized, that the resulting chiral building blocks, can be used in subsequent coupling reactions to prepare oligoprenoids of any desired length and stereochemistry. This novel approach should, therefore, show broad application in the total synthesis of a range of natural products and analogues thereof.
To demonstrate the synthetic versatility of this catalytic approach, it was employed in the total synthesis (Scheme 2) of two—female-produced—pheromones 11 and 12 of the apple leafminer (Lyonetia prunifoliella), a pest endemic to the eastern regions of North America.17,18 The total synthesis required, that both ends of the anti-building block (1a) are elongated with alkyl chains. As a first step, the primary alcohol was converted into the tosylate 7 in 95% yield. Crude 7 was then treated with DIBAH to give the primary alcohol 8 in 94% yield. Subsequent chain elongation by reaction with a large excess of n-propylmagnesium bromide (16 eq.) in the presence of a stoichiometric amount of CuBr·SMe2 gave 9 in excellent yield (99%).19 After conversion of the hydroxyl moiety of 9 into a tosyl group (10, 94%), the product was applied in coupling reactions with 6-heptenylmagnesium bromide and hexylmagnesium bromide in the presence of CuBr·SMe2 to give 11 and 12, respectively, with full conversion.20 Further applications of this strategy are currently under investigation in our laboratory.
We would like to thank T.D. Tiemersma-Wegman (GC) and A. Kiewiet (MS) for technical support. This work was supported by the Dutch Organization for Scientific Research (NWO).
Notes and references
- (a) K. Mori, in The Total Synthesis of Natural Products, ed. J. ApSimon, Wiley-Interscience, New York, 1992, 9 Search PubMed; (b) Y. Nakamura and K. Mori, Eur. J. Org. Chem., 1999, 2175 CrossRef CAS; (c) J. R. Aldrich, J. E. Oliver, W. R. Lusby, J. P. Kochansky and M. J. Borges, J. Chem. Ecol., 1994, 20, 1103 CAS.
- (a) C. Mercier and P. Chabardes, Pure Appl. Chem., 1994, 66, 1509 CrossRef CAS; (b) P. Dowd, R. Hershline, S. W. Ham and S. Naganathan, Nat. Prod. Rep., 1994, 11, 251 RSC.
- (a) L. Minale, in Marine Natural Products, ed. P. J. Scheuer, Academic Press, 1978, vol. 1, ch. 4 Search PubMed; (b) S. Sankaranarayanan, A. Sharma and S. Chattopadhyay, Tetrahedron: Asymmetry, 2002, 13, 1373 CrossRef.
- (a) D. Zhang and C. D. Poulter, J. Am. Chem. Soc., 1993, 115, 1270 CrossRef CAS; (b) W. F. Berkowitz and Y. Wu, J. Org. Chem., 1997, 62, 1536 CrossRef CAS.
- (a) T. Eguchi, K. Arakawa, T. Terachi and K. Kakinuma, J. Org. Chem., 1997, 62, 1924 CrossRef CAS; (b) S. Hanessian, P. J. Murray and S. P. Sahoo, Tetrahedron Lett., 1985, 26, 5623 CrossRef CAS.
- R. Chênevert and M. Desjardins, J. Org. Chem., 1996, 61, 1219 CrossRef CAS.
- (a) C. H. Heathcock, B. L. Finkelstein, E. T. Jarvi, P. A. Radel and C. R. Hadley, J. Org. Chem., 1988, 53, 1922 CrossRef CAS; (b) J. Fujiwara, Y. Fukutani, M. Hasegawa, K. Maruoka and H. Yamamoto, J. Am. Chem. Soc., 1984, 106, 5004 CrossRef CAS; (c) A. Gambacorta, D. Tofani, P. Lupattelli and A. Tafi, Tetrahedron Lett., 2002, 43, 2195 CrossRef CAS.
- H. Takaya, T. Ohta, N. Sayo, H. Kumobayashi, S. Akutagawa, S. Inoue, I. Kasahara and R. Noyori, J. Am. Chem. Soc., 1987, 109, 1596 CrossRef CAS.
- S. Huo, J. Shi and E. Negishi, Angew. Chem. Int. Ed., 2002, 41, 2141 CrossRef CAS.
- (a) B. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos and A. H. M. De Vries, Angew. Chem. Int. Ed., 1997, 36, 2620 CrossRef CAS; (b) B. L. Feringa, Acc. Chem. Res., 2000, 33, 346 CrossRef CAS; (c) R. B. C. Jagt, R. Imbos, R. Naasz, A. J. Minnaard and B. L. Feringa, Isr. J. Chem., 2001, 41, 221 CAS.
- O. Knopff and A. Alexakis, Org. Lett., 2002, 4, 3835 CrossRef CAS.
- K. C. Nicolaou, T. Montagnon, P. S. Baran and Y.-L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245 CrossRef CAS.
- Partial racemization of the cis-adduct 5b upon use of TMSOTf is probably due to the fact that TMSOTf is reactive enough to convert small quantities of (meso) ketone into racemic 5b while TMSCl is not.
- The main loss of product occurred in the purification of 5a/5b due to volatility of the silyl enol ethers. Therefore, CH2Cl2 was the solvent of choice in the second 1,4-addition, even though toluene gave similar conversions and equally high ee's and de's.
- In this case the prime loss of product was due to oxidation of the aldehyde in the ozonolysis step.
- (7S)- 3, (3S,7S)-1a and (3S,7R)-1b were isolated in comparable yield, ee and de as compared to their enantiomers.
- For earlier synthesis of these pheromones see: Y. Nakamura and K. Mori, Eur. J. Org. Chem., 2000, 2745 Search PubMed.
- R. Gries, G. Gries, G. G. S. King and C. T. Maier, J. Chem. Ecol., 1997, 23, 1119 CrossRef CAS.
- The use of catalytic amounts of CuBr·SMe2 or Li2CuCl4 and a smaller excess of Grignard reagent (5 eq.) was less successful, with incomplete reaction after 12 h and partial exchange of the tosyl group by bromide.
- 12 was not separated from dodecane, which resulted from homocoupling of the Grignard. The estimated yield (GC) of 12 was 70%.
Footnote |
| † Electronic supplementary information (ESI) available: detailed experimental procedures and spectroscopic (1H and 13C NMR) and analytical data of all compounds in Schemes 1 and 2. Chiral GC-methods for determination of ee and de of compounds 3 and 5. See http://www.rsc.org/suppdata/cc/b4/b419268k/ |
| This journal is © The Royal Society of Chemistry 2005 |
