Direct, trace level detection of explosives on ambient surfaces by desorption electrospray ionization mass spectrometry
Zoltán
Takáts
,
Ismael
Cotte-Rodriguez
,
Nari
Talaty
,
Huanwen
Chen
and
R. Graham
Cooks
*
Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA
First published on 7th February 2005
Abstract
Desorption electrospray ionization (DESI) mass spectrometry is used to detect trace amounts of explosives present on a variety of ambient surfaces in 5-second analysis times without any sample preparation.
Detection of explosives is a subject of continuing strong interest in analytical chemistry, driven by threats to civil society1 and by environmental problems2 associated with explosives residues. The requirements of an ideal method include (i) high sensitivity, (ii) applicability to involatile and thermally unstable analytes, (iii) high specificity to minimize the chance of false positives or false negatives (iv) rapid response times and (v) no sample preparation or handling. No methods currently available meet all these criteria.
Ion mobility spectrometry (IMS) has been a common choice for addressing this problem; it has the advantage of high sensitivity and speed, but suffers in terms of the other criteria. Mass spectrometry is widely considered to have the best specificity of any technique applicable to the broad class of explosive compounds and it is highly sensitive but until now it has required significant sample manipulation. Another barrier to the use of mass spectrometry is that some of the explosives are non-volatile compounds which are not easily ionized by traditional methods. Although a wide variety of desorption ionization methods is available for the MS analysis of compounds on surfaces3 they require operation under vacuum conditions. Since traditional desorption ionization methods fail at in-situ explosives detection, the approach usually pursued involves wiping the ambient surface with a special material wipe followed by thermal desorption/gas phase ionization of the explosive compounds.4–8 Although this dry sampling/thermal method is widely employed in airport explosive detection systems, it requires manual sample transfer, is relatively slow and is not ideal for the detection of thermally labile explosives or explosives which have low vapor pressures.9 The requirement for sample manipulation is also a disadvantage of solution phase mass spectrometry methods of analysis based on electrospray ionization. This is unfortunate because most explosives show high affinities for various anions and can be ionized directly by electrospray ionization10–13 or by gas-phase anion attachment, typically using anions generated by electrospray.14 The high electron affinities associated with the nitro- or nitrate functional groups present in the overwhelming majority of explosives in common use means that they readily form negative ions by electron capture. Various electron sources including corona discharge,15 glow discharge16,17 and 63Ni beta emitters have been successfully implemented as ion sources for explosive detection, including the direct detection of explosives in air.
The recently developed DESI method18 is performed by directing a pneumatically-assisted electrospray onto a surface bearing an analyte and collecting the secondary ions generated by the interaction of the charged microdroplets from the electrospray with the neutral molecules of the analyte present on the surface. The main advantage of DESI compared to other desorption ionization methods is that it does not require sample pretreatment and it can be performed under ambient conditions from any common surface. As is shown in this Communication, the method retains the advantages of sensitivity and specificity of other mass spectrometry methods and combines these with very short (5 s) total analysis times, no sample preparation at all and the ability to detect explosives in situ on virtually any surface.
DESI mass spectra of various explosives (TNT, PETN, RDX, HMX) were recorded using methanol/water or ethanol/water sprays.19 Source parameters are summarized elsewhere.18 Ions were sampled and analyzed using a Thermo Finnigan LTQ mass spectrometer in both the positive and negative ion modes. Observed ions, and LOD's for the different explosives, are summarized in Table 1. LOD's refer to the total amount of analyte present on the surface and to a 3 : 1 signal-to-noise ratio. In referring to Table 1, note that the explosives were determined on several different surfaces—including paper, plastic and metal—and that these experiments were performed without any sample preparation or manipulation.
| Explosive | ± | Ions observed | LOD | S/N at LOD |
|---|---|---|---|---|
| a Data covers a number of different surfaces for each explosive, including plastic, metal and paper b A anion: Cl−, acetate, trifluoroacetate, nitrate c Paper. d Metal. e Plastic. f Paper, metal and plastic. | ||||
| TNT | − | M−˙, (M − NO)− | <1 pge | 12 : 1 |
| RDX | + | (M + H)+ | <1 pgc | 4 : 1 |
| RDX | − | (M + A)−b | <10 pgf | 9 : 1 |
| HMX | − | (M + A)−b | <10 pgd | 3 : 1 |
| PETN | − | (M − H)− | <100 pgc | 5 : 1 |
Most explosives give best DESI performance in the negative ion mode. In this mode, TNT undergoes electron capture (EC) ionization yielding the radical anion at m/z 227, Fig. 1. This ion undergoes characteristic losses of NO and OH in the MS/MS experiment (inset in Fig. 1). Detection limits in the range 10–100 fg were achieved. The signal intensity was dependent on the voltage applied to the electrospray source, strongly implicating the corona discharge accompanying ESI as the primary source of electrons for EC ionization. In the positive ion mode, TNT does not give a signal with the methanol/water spray solution, probably because its proton affinity is much lower than that of methanol.
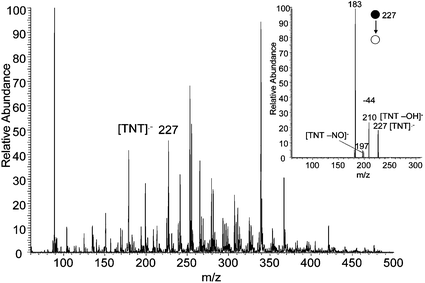 | ||
| Fig. 1 Negative ion DESI spectrum of 10 fg TNT on a paper surface. Methanol/water 1 : 1 was used as the spray and a 4 mm2 area was sampled. The corresponding MS/MS product ion spectrum of the molecular radical anion, m/z 227, is shown in the inset. | ||
Pentaerythritol-tetranitrate (PETN), a representative polyol-nitrate explosive, gives (M − H)− and (M − NO3)− ions in negative ion DESI. The limit of detection of PETN from paper was 100 pg. Note that all the explosives were successfully detected from paper, a surface which is impossible to sample at these levels using other direct analytical methods. Normally, thermal evaporation is used for this type of surface4–8 but this involves a sample manipulation step absent in DESI. Note also that explosives such as RDX and TNT can be detected directly on skin using DESI.
The aliphatic nitrocompounds RDX and HMX were observed to give a wide variety of low abundance anion adducts when pure methanol/water was used as the spray solution in the negative ion mode. However, specific adducts are formed in high abundance by addition of an appropriate reagent into the sprayed solvent. Fig. 2 depicts the negative ion DESI mass spectrum of HMX using methanol/water containing hydrochloric acid. Anionic adducts of both HMX and RDX with chloride, acetate or trifluoroacetate can be formed using the appropriately modified spray solution and each dissociates in the MS/MS experiment to yield the original anion and the neutral explosive molecule. The previously reported10 NNO3 adducts of both species show a characteristic fragmentation involving loss of oxygen and various nitrogen oxides in DESI.
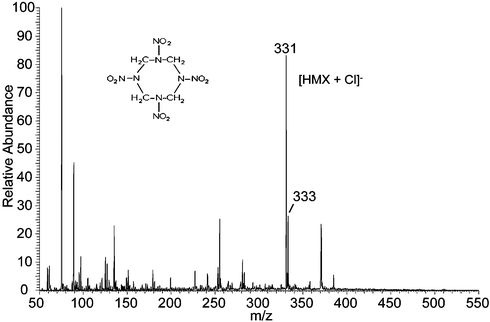 | ||
| Fig. 2 Negative ion DESI spectrum of 10 pg HMX on a metal surface. Methanol/water 1 : 1 containing 0.05% HCl was used as the spray and a 4 mm2 area was sampled. | ||
Significantly, RDX also gives excellent DESI spectra in the positive ion mode using methanol/water as the spray solvent, Fig. 3. The main ionic species formed in these cases is the protonated molecule. Besides (M + H)+ ions, ionic species 14 and 28 higher in m/z ratio were also detected and these species are tentatively identified as the alkylated molecules, where the alkyl residues originate from the methanol solvent. Protonated RDX shows a characteristic fragmentation pattern in MS/MS as is shown in the inset to Fig. 3. Although the ionization mechanism of RDX in the positive mode is not yet clear, and HMX does not show similar characteristics, positive ion DESI is a highly sensitive as well as a highly specific technique for the detection of RDX. The fact that this important explosive can be characterized at low levels in both the positive and negative ion modes adds to the specificity of the DESI analysis.
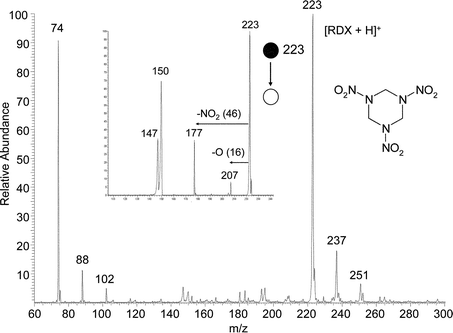 | ||
| Fig. 3 Positive ion DESI spectrum of 10 pg RDX present on paper. Low mass ions are associated with decomposition products of RDX. Methanol/water 1 : 1 was used as the spray and a 4 mm2 area was sampled. The corresponding MS/MS product ion spectrum of the protonated molecule, m/z 223, is shown in the inset. | ||
Ion formation in DESI in the case of explosives may involve two different mechanisms. One involves the formation of charged solvent species in the electrospray source, followed by ionization of the analyte molecule on the surface by charge transfer. The last step in this case, the desorption of analyte ions from the surface, is a type of chemical sputtering.20 Static charge accumulation on the insulating surface might facilitate ejection of the analyte ion. The alternative mechanism involves the impact of electrosprayed droplets on the surface, dissolution of the analyte in the droplet, and subsequent evaporation by mechanisms well known in ESI.
More insight into the mechanism of the ionization process was obtained in an experiment in which only the first ionization mechanism can occur. To achieve this, the electrospray emitter in the DESI source was replaced by a tapered tip stainless steel needle, and vapors of various solvents were mixed into the annular gas flow. A high voltage, from 3 to 6 kV, was applied to the steel needle. The gaseous solvent vapors were ionized in close proximity to the stainless steel tip by corona discharge ionization. Since droplets are not formed in this case, the second ionization mechanism can be excluded. This experiment, termed desorption atmospheric pressure chemical ionization (DAPCI), shows similar results to DESI in those cases where electron attachment or protonation/deprotonation is the main ionization mechanism. For example, TNT gives M−˙ ions if toluene or methanol vapors are used as reagent gases. PETN also gave M− and (M − H)− when toluene or methanol was used. However, RDX showed DESI-like spectra in the positive ion mode with a wide variety of reagent vapors, but the formation of negative adduct ions was not observed using DAPCI with acetic acid, trifluoroacetic acid or dichloromethane (as a chloride ion source). These results imply that in most cases ionization follows the mechanism in which reagent ions are formed in the electrospray and these reagent ions ionize the analyte molecules by either electron or proton transfer in a thermochemically-controlled chemical ionization step. The reagent ions blanket the surface causing static charge build-up which facilitates ion desorption and transport towards the mass spectrometer. On the other hand, formation of anionic adducts in the case of RDX and HMX is more reasonably ascribed to the droplet pick-up mechanism.
The results obtained show that DESI can be used to detect traces of explosives present on surfaces including paper, plastic, glass, leather and skin. The method is fast (it takes a few seconds to obtain spectra), highly selective and its detection limits are sub-nanogram in all of the studied cases, and sub-picogram in the case of TNT. It is applicable to explosives in complex mixtures, including diesel oil and on complex surfaces including brick and concrete. In separate experiments, sampling rates of up to 10 per second have been achieved in DESI analysis.21 When DESI is coupled to a portable mass spectrometer it should revolutionize transportation security.
This work was funded by the National Science Foundation (0412782), ONR and InProteo, LLC, Indianapolis.
Notes and references
- N. M. A. Board, National Academy of Sciences, Washington, D.C., 2004.
- T. A. Lewis, D. A. Newcombe and R. L. Crawford, J. Environ. Manage., 2004, 70, 291–307 Search PubMed.
- K. L. Busch, J. Mass Spectrom., 1995, 30, 233–240 CAS.
- D. D. Fetterolf and T. D. Clark, J. Forensic Sci., 1993, 38, 28–39 CAS.
- R. Waddell, D. E. Dale, M. Mongale and S. A. Smith, J. Chromatogr., A, 2005, 1062, 125–131 CrossRef CAS.
- P. Kolla, Anal. Chem., 1995, 67, 184A CAS.
- M. E. Sigman and C.-Y. Ma, Anal. Chem., 1999, 71, 4119–4124 CrossRef CAS.
- J. I. Steinfeld and J. Wormhaudt, Annu. Rev. Phys. Chem., 1998, 49, 203–232 CrossRef CAS.
- T. L. Buxton and P. D. Harrington, Anal. Chim. Acta, 2001, 434, 269–282 CrossRef CAS.
- J. Yinon, J. E. McClellan and R. A. Yost, Rapid Commun. Mass Spectrom., 1997, 11, 1961–1970 CrossRef CAS.
- G. R. Asbury, J. Klasmeier and H. H. Hill, Talanta, 2000, 50, 1291–1298 CrossRef.
- B. Casetta and F. Garofolo, Org. Mass Spectrom., 1994, 29, 517–525 CAS.
- K. A. Daum, D. A. Atkinson and R. G. Ewing, Talanta, 2001, 55, 491–500 CrossRef CAS.
- M. Tam and H. H. Hill, Anal. Chem., 2004, 76, 2741–2747 CrossRef CAS.
- M. Tabrizchi and A. Abedi, Int. J. Mass Spectrom., 2002, 218, 75–85 CrossRef CAS.
- S. A. Mcluckey, G. L. Glish, K. G. Asano and B. C. Grant, Anal. Chem., 1988, 60, 2220–2227 CrossRef CAS.
- J. G. Zhao, J. Z. Zhu and D. M. Lubman, Anal. Chem., 1992, 64, 1426–1433 CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Science, 2004, 306, 471–473 CrossRef CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Anal. Chem., 2004, 76, 4050–4058 CrossRef CAS.
- T. Ast, D. E. Riederer, S. A. Miller, M. Morris and R. G. Cooks, Org. Mass Spectrom., 1993, 28, 1021–1033 CAS.
- H. C. Chen, N. Talaty, Z. Takats and R. G. Cooks, unpublished.
| This journal is © The Royal Society of Chemistry 2005 |
