A metal–organic gel used as a template for a porous organic polymer
Qiang
Wei
and
Stuart L.
James
*
Centre for the Theory and Application of Catalysis, School of Chemistry, Queen's University Belfast, David Keir Building, Stranmillis Road, Belfast, Northern Ireland, UK BT9 5AG. E-mail: q.wei@qub.ac.uk; s.james@qub.ac.uk
First published on 4th February 2005
Abstract
A polymeric metal–organic gel is described, which acts as a template in the preparation of macroporous polymethylmethacrylate, and can be easily removed post-polymerisation.
Coordination polymers, or metal–organic frameworks (MOFs), in which metal ions are linked together by organic bridging ligands, have developed extremely rapidly in recent years.1 These materials have caused great excitement due to their diverse and, to some degree, controllable, structures and because of their unusual and useful inclusion properties. However, the investigation and exploitation of their structures has generally been at the molecular level and comparatively little is known about how their larger-scale structure may be controlled and used.2 Coordination polymers are also normally prepared and characterised in the crystalline state, partly because of the detailed structural information that can be gained from single-crystal X-ray crystallography. Consequently, much less is known about coordination polymer gels,3 despite their many possibilities as catalytic materials,3a absorbents, sensors3b and other responsive materials, endowed with complex behaviours by the presence of metal centres. Here we report on a previously unexplored aspect of this unusual type of material, specifically an iron–organic gel whose macroscopic structure enables it to be used as a template for the formation of a macroporous organic polymer.
Reaction between Fe(NO3)3·9H2O and 1,3,5-benzenetricarboxylic acid (H3BTC) in a 3 : 2 molar ratio ethanol solvent rapidly gives a gel within 3 min, of which the solvent content can be varied from 95 to 98% by weight, depending on the ratio of reagents to solvent used.4 The gelation of the metal and ligand suggests that there is rapid cross-linking polymerisation between Fe3+ and the tricarboxylic acid, leading to the growth of coordination polymer particles, which themselves subsequently cross-link to leave macroscopic solvent-filled cavities. A similar gel is obtained in methanol, or on heating Fe(NO3)3·9H2O and H3BTC in DMF. The gel is stable when subsequently immersed in common solvents (alcohols, DMSO, DMF, acetone, CH2Cl2) or in water but dissolves in 1 M hydrochloric acid. The gel is apparently stable if sealed so that its solvent content cannot evaporate, however, when open to the air it shrinks markedly over a period of 3–6 days.
Interestingly, the gel will also form in the presence of organic polymer precursors. If the metal–organic polymerisation is conducted in a 1 : 1 (v/v) mixture of ethanol and methylmethacrylate (MMA), containing some azoisobutyronitrile (AIBN), a gel with a similar appearance is obtained. UV irradiation for 30 h then causes polymerisation of the MMA to give PMMA. The Fe–BTC content of this orange material can be removed by washing with hydrochloric acid to leave a colourless polymethylmethacrylate (PMMA) powder, which is expected to have a structure which is a porous ‘imprint’5 of the metal–organic gel (Fig. 1). The porosity of the organic polymer was confirmed by SEM (Fig. 2). The images reveal a sponge-like structure with a disordered arrangement of interconnected pores in the size range ca. 1–10 μm.
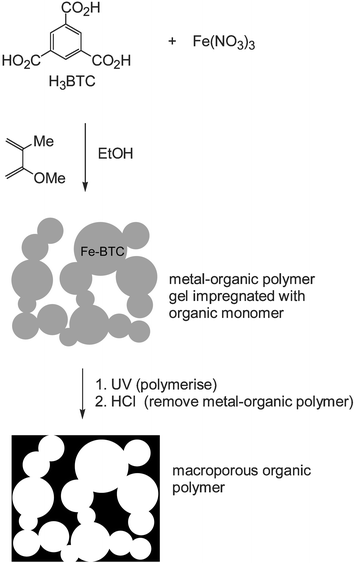 | ||
| Fig. 1 Schematic representing the formation of a metal–organic gel and its use as a template for a porous organic polymer. | ||
 | ||
| Fig. 2 SEM images of sponge-like polymethylmethacrylate after washing away the Fe–BTC template. | ||
Templated porous organic polymers, including ‘polyHIPES’ have potential applications in separations and as supports. Previous methods used to template them include the use of CO2–water emulsions,6 polymer particle emulsions7 and suspended silica particles.8 To our knowledge, this is the first use of a metal coordination polymer template, and it has the advantage of being a relatively straightforward and inexpensive procedure. In addition, the presence of metal–organic polymer particles within an organic polymer suggests that a range of responsive and magnetic9 materials may be developed using this approach.
In summary, we report the formation of an iron–organic gel, its hybridisation with an organic polymer, and subsequent removal of the metal–organic gel to leave a porous imprint on the organic polymer. Further studies into the macroporosity and magnetic properties of these materials are in progress.†
We are grateful to the Questor Centre, Belfast for funding (Q.W.).
Notes and references
- S. L. James, Chem. Soc. Rev., 2003, 32, 276 RSC; C. Janiak, J. Chem. Soc., Dalton Trans., 2003, 2781 RSC; M. J. Rosseinsky, Microporous Mesoporous Mater., 2004, 73, 15 CrossRef CAS; J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3 CrossRef CAS; B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; M. J. Zaworotko, Angew. Chem., Int. Ed., 2000, 39, 3052 CrossRef CAS; B. Moulton and M. J. Zaworotko, Curr. Opin. Solid State Mater. Sci., 2002, 6, 117 CrossRef CAS; M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS; M. J. Zaworotko, Nature, 1999, 402, 242 CrossRef CAS; A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk and M. Schröder, Coord. Chem. Rev., 2001, 222, 155 CrossRef CAS; C. Janiak, Angew. Chem., Int. Ed. Engl., 1997, 36, 1431 CrossRef CAS; O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS.
- For stabilisation of MOF nanoparticles see M. Yamada, M. Arai, M. Kurihara, M. Sakamoto and M. Miyake, J. Am. Chem. Soc., 2004, 126, 9482 Search PubMed.
- (a) B. Xing, M.-F. Choi and B. Xu, Chem., Eur. J., 2002, 8, 5028 CrossRef CAS; (b) B. Xing, M.-F. Choi and B. Xu, Chem. Commun., 2002, 368 RSCB. A. Xing, M. F. Choi, Z. Y. Zhou and B. Xu, Langmuir, 2002, 18, 9654 Search PubMed; J. B. Beck and S. J. Rowan, J. Am. Chem. Soc., 2003, 125, 13922 Search PubMed.
- The formation of a transient Fe(III)–organic gel prior to the formation of malonate complexes has previously been mentioned in C. T. Daziobkowski, J. T. Wrobleski and D. B. Brown, Inorg. Chem., 1981, 20, 671. Search PubMed A series of Fe(II)–BTC complexes has been reported by Ferey et al., M. Riou-Cavallec, C. Albinet, C. Livage, N. Guillou, A. Nogues, J. M. Greneche and G. Ferey, Solid State Sci., 2002, 4, 267; M. Riou-Cavellec, C. Albinet, J. M. Greneche and G. Ferey, J. Mater. Chem., 2001, 11, 3166 Search PubMed.
- R. J. H. Hafkamp, B. P. A. Kokke, I. M. Danke, H. P. M. Geurts, A. E. Rowan, M. C. Feiters and R. J. M. Nolte, Chem. Commun., 1997, 545 RSC; W. Gu, L. Lu, G. B. Chapman and R. G. Weiss, Chem. Commun., 1997, 543 RSC.
- R. Butler, I. Hopkinson and A. I. Cooper, J. Am. Chem. Soc., 2003, 125, 14473 CrossRef CAS.
- R. Mezzenga, J. Ruokolainen, G. H. Fredrickson and E. J. Kramer, Macromolecules, 2003, 36, 4466 CrossRef CAS.
- S. A. Johnson, P. J. Ollivier and T. E. Mallouk, Science, 1999, 283, 963 CrossRef CAS.
- S. Frank and P. C. Lauterbur, Nature, 1993, 363, 334 CrossRef CAS; D. Collin, G. K. Auernhammer, O. Gavat, P. Martinoty and H. R. Brand, Macromol. Rapid Commun., 2003, 24, 737 CrossRef CAS.
Footnote |
| † Preparation of Fe–BTC gel: Fe(NO3)3·9H2O (1.21 g, 3.0 mmol) and H3BTC (0.42 g, 2.0 mmol) were each dissolved in ethanol (20 ml each), and the two solutions rapidly mixed together for 5 s, then left to stand. A yellow–brown gel was obtained after less than 1 min (wt% ∼5%). Preparation of porous PMMA: H3BTC (0.105 g, 0.5 mmol), AIBN (0.20 g, 1.2 mmol) and MMA (5.0 ml, 4.68 g, 46.7 mmol) were dissolved in 5 ml ethanol. This solution was added to a solution of Fe(NO3)3·9H2O (0.30 g, 3 mmol) in ethanol (5 ml) and the mixture left to stand. A yellow–brown gel was obtained in 3 min. The gel was irradiated with UV light (365 nm) for 30 h, then washed repeatedly with HCl solution (1 M) to leave a white solid which was collected by filtration, washed repeatedly with water (200 ml) and dried at 88 °C overnight. Yield: 2.0 g. FT-IR (KBr, cm−1): 3399.4(br), 3018(m), 2842(m), 1613(vs), 1601(vs), 1518(vs), 1465(s), 1407(m), 1315(s), 1249(s), 1180(s), 1118(m), 1073(m), 990(w), 813(s), 751(s), 692(m), 509(w). |
| This journal is © The Royal Society of Chemistry 2005 |
