Ionic liquid crystal as a hole transport layer of dye-sensitized solar cells
Noriyo Yamanakaa, Ryuji Kawanob, Wataru Kuboa, Takayuki Kitamuraa, Yuji Wadaa, Masayoshi Watanabeb and Shozo Yanagida*c
aMaterial and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka Suita, 565-0871, Japan. E-mail: ywada@mls.eng.osaka-u.ac.jp
bDepartment of Chemistry and Biotechnology, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. E-mail: mwatanab@ynu.ac.jp
cCenter for Advance Science and Innovation Osaka University, Japan. E-mail: yanagida@mls.eng.osaka-u.ac.jp
First published on 12th January 2005
Abstract
Use of a new ionic liquid crystal, 1-dodecyl-3-methylimidazolium iodide, and iodine as an electrolyte of dye-sensitized solar cells leads to a high short circuit photocurrent density and a high light-to-electricity conversion efficiency, due to a self-assembled structure of the imidazolium cations, resulting in high conductivity of the electrolyte.
Dye-sensitized solar cells (DSSC), constructed by using dye molecules, nanocrystalline metal oxides and liquid electrolytes, have attractive features in terms of the high light-to-electricity conversion efficiency, and the low production cost and energy.1 The electrolytes, usually composed of an I−/I3− redox couple in organic solvents, are sealed between two electrodes. These organic solvents cause a serious problem of low durability due to evaporation.2 It has been reported that DSSC using ionic liquids as a non-volatile solvent achieves high temperature stability.3,4 However, the conversion efficiency of the cells using ionic liquids is lower than that using organic solvents, because the high viscosity of the ionic liquids retards the physical diffusion of I− and I3−. Many attempts to reduce the viscosity have not yet been successful.5 For enhancing the conductivity of ionic liquids leading to the high light-to-electricity conversion efficiency of DSSC, it seems to be necessary to arrange a pathway for fast charge transport.
Previously, we reported that the charge transport rate at high concentration of an I−/I3− redox couple in ionic liquids can be attributed to the exchange reaction of I− + I3− → I3− + I−.4,6 For enhancing the short circuit photocurrent density (JSC) of DSSC using ionic liquids with a high concentration of an I−/I3− redox couple, the exchange reaction in the ionic liquids needs to be promoted. In this study, we report a new strategy for enhancing the conductivity of ionic liquid electrolytes; employing an ionic liquid crystal (ILC) as a constituent of an electrolyte, which forms a self-assembled structure and promotes the exchange reaction by the locally increased concentrations of I− and I3−. We have selected 1-dodecyl-3-methylimidazolium iodide (C12MImI) as an ILC. This provides a self-assembled structure of the imidazolium cations like a solid, while maintaining the molecular dynamics like a liquid. The ILC with the smectic A phase (SA) has a bilayer structure of interdigitated alkyl chains of the imidazolium cations, and I− and I3− would be localized between the SA layers. The locally high concentration would promote the exchange reaction. So, the ILC with the SA phase would be suitable for the electrolyte of DSSC when aiming at the high light-to-electricity conversion efficiency.
A few examples of the ILC with the SA phase, such as imidazolium salts consisting of cations with alkyl chains of C12–C18 and anions of hexafluorophosphate or bromide, have been reported.7,8 Iodide is indispensable in a DSSC electrolyte, because the I−/I3− redox couple functions as a hole transport agent. However, an imidazolium salt with iodide as the counter anion has not been reported to be an ILC with a SA phase. Here, we show for the first time that imidazolium iodides with alkyl chains longer than C12 exhibit a SA phase and that the liquid crystalline nature is preferable in terms of the hole transport layer in DSSC.
Imidazolium iodides with long alkyl chains were synthesized by the quaternization reaction of 1-methylimidazole with an equimolar amount of the corresponding alkyl iodide for 72 h under N2 atmosphere at room temperature. The products were washed with n-hexane to remove the remaining starting materials and dried under vacuum at 40 °C for 4 h, and finally identified by 1H NMR in CDCl3 and differential scanning calorimetry (DSC). Imidazolium iodides with alkyl chains longer than C12 showed a liquid crystalline phase. C12MImI showed the lowest melting point and viscosity among them and these properties were suitable for the hole transport layer in DSSC. In this study, we selected C12MImI and applied it with 0.65 M iodine (C12MImI/I2) as the hole transport layer in DSSC. We have compared the properties of C12MImI/I2 with an ionic liquid electrolyte; 1-undecyl-3-methylimidazolium iodide with 0.65 M iodine (C11MImI/I2).
C12MImI showed a phase transition from a liquid to a liquid crystal at 80 °C, although C11MImI did not show a liquid crystalline phase. The ionic liquid crystalline phase of C12MImI was confirmed by polarized optical microscopy (POM). The characteristic focal conic domains observed during the cooling process suggested that the liquid crystalline phase of C12MImI was a SA phase.7
Photoelectrochemical cells were fabricated as previously described.9 C11MImI/I2 and C12MImI/I2 were used as the hole transport layer, respectively. C12MImI/I2 maintained the SA phase ranging from 27 to 45 °C on heating. On the other hand, C11MImI/I2 showed a liquid phase above 37 °C.
The light-to-electricity conversion efficiencies of DSSC using C12MImI/I2 and C11MImI/I2 were evaluated at 40 °C under AM 1.5 irradiation from a solar simulator, adaptable for amorphous silicon solar cells according to the Japanese Industrial Standard.10 Each value for cell performance was taken as an average of at least 3 samples.
Fig. 1 shows photocurrent–voltage curves of the DSSC using C12MImI/I2 and C11MImI/I2. JSC of the DSSC using C12MImI/I2 was higher than that using C11MImI/I2, while the open circuit voltage (VOC) and fill factor (FF) were the almost same values as for C11MImI/I2. This result suggests that the higher conductivity of C12MImI/I2 than C11MImI/I2 could lead to a high JSC.11,12
 | ||
| Fig. 1 Photocurrent–voltage curves of the cells using C12MImI/I2 (bold solid curve) and C11MImI/I2 (solid curve) under AM 1.5 irradiation. | ||
To examine the conductivities of C12MImI/I2 and C11MImI/I2, the diffusion-limited currents (Ilim) corresponding to the reaction of I3− + 2e− → 3I− were measured. The measurements were carried out at 40 °C by using a microelectrode and the diffusion coefficients (D) of I3− were calculated using the values of Ilim as previously described.6 According to this study, the D value calculated from Ilim intrinsically includes not only the simple physical diffusion coefficient but also the diffusion coefficient based on the exchange reaction.
The observed D value of C12MImI/I2 (4.2 × 10−8 cm2 s−1) was 1.3 times as large as that of C11MImI/I2 (3.2 × 10−8 cm2 s−1), while the viscosity of C12MImI/I2 was 2.5 times larger than that of C11MImI/I2. The simple physical diffusion coefficient of C12MImI/I2 should be smaller than C11MImI/I2. This result would lead to the idea that the diffusion coefficient based on the exchange reaction is increased in C12MImI/I2. It has been reported that anisotropic long-range conductive pathways between the SA layers are formed in liquid crystals with a SA phase.13 In C12MImI/I2, these pathways should be formed, and I− and I3− should be located between the SA layers consisting of the imidazolium cations. If a locally high concentration of I− and I3− is achieved in the pathways, the exchange reaction should be promoted in C12MImI/I2, since the diffusion coefficient value based on the exchange reaction is proportional to the concentrations of I− and I3−. So, C12MImI/I2 could show a higher D value than C11MImI/I2 in which the cations should exist at random.
To prove that the exchange reaction was promoted in the pathways between the SA layers of C12MImI/I2, we measured the ionic conductivities along the direction parallel (σi∥) and perpendicular (σi⊥) to the SA layer plane13 at temperatures ranging from 32.5 to 57.5 °C. A glass plate with comb-shaped platinum electrodes (cell A) and a pair of indium tin oxide (ITO) electrodes (cell B) were employed for the measurements of σi∥ and σi⊥, respectively. The conoscopic image at 40 °C for C12MImI/I2 revealed that the self-assemblies of the cations formed a homeotropic alignment in the SA phase on the glass surface.
Fig. 2 shows anisotropic ionic conductivities of C12MImI/I2 and isotropic ionic conductivities of C11MImI/I2 as a function of temperature. It was reported that the ionic conductivities of isotropic ionic liquids gradually increased with the increase in temperature as shown in the insert of Fig. 2.14 In contrast, for C12MImI/I2, the discontinuous changes of ionic conductivities were observed. The ionic conductivities parallel (σi∥) to the SA layer of C12MImI/I2 decreased and those perpendicular (σi⊥) increased at 45 °C on heating. When the liquid crystalline phase-order disappeared above the phase transition temperature, the ionic conductivities measured in both cell A and cell B were on the same line within the limits of error. Taking into account that C11MImI/I2 did not show any discontinuous changes in σi∥ and σi⊥ over the whole temperature range, the discontinuous changes of C12MImI/I2 should be attributed to the conductive pathways formed between the SA layers. The observed σi∥ values of C12MImI/I2 below 45 °C, which showed ionic conductivities along the direction of the conductive pathways, were enhanced because the exchange reaction would be promoted at the conductive pathways.
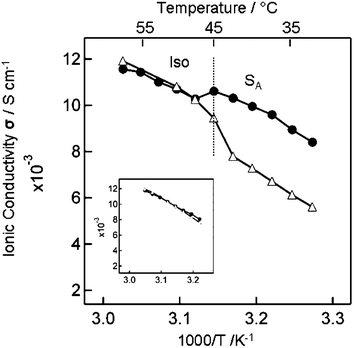 | ||
| Fig. 2 Ionic conductivities for the samples of C12MImI/I2 and C11MImI/I2 (insert) along with the direction parallel σi∥ (filled circles) and perpendicular σi⊥ (open triangles) to the SA layer plane of the homeotropically aligned ionic liquid crystal. | ||
In the DSSC using C12MImI/I2, although the long-range order of ILC was not necessarily achieved in the mesoporous TiO2 electrode, the localization of I− and I3− between the SA layers of each domain should result in the promotion of the exchange reaction in a hole transport layer and the enhancement in JSC.
In conclusion, we have demonstrated a new strategy for enhancing the conductivity of the ionic liquid electrolytes containing an I−/I3− redox couple. This strategy is based on the promotion of the exchange reaction between I− and I3− by the locally increased concentrations of I− and I3−. As a means of demonstrating this strategy, a new ionic liquid crystalline C12MImI/I2 with a self-assembled structure of the imidazolium cations was introduced. C12MImI/I2 showed a high ionic conductivity in spite of its high viscosity. The ionic liquid crystalline hole transport layer (C12MImI/I2) was applied to DSSC, which showed a higher JSC and a higher light-to-electricity conversion efficiency than that using the ionic liquid hole transport layer (C11MImI/I2).
This research was supported in part by the New Energy and Industrial Technology Development Organization (NEDO) under the Ministry of Economy, Trade and Industry.
Notes and references
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. H. Baker, E. Mueller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS.
- A. Kay and M. Grätzel, Sol. Energy Mater. Sol. Cells., 1996, 44, 99 CrossRef CAS.
- W. Kubo, T. Kitamura, K. Hanabusa, Y. Wada and S. Yanagida, Chem. Comun., 2002, 374 Search PubMed.
- W. Kubo, K. Murakoshi, T. Kitamura, S. Yoshida, M. Haruki, K. Hanabusa, H. Shirai, Y. Wada and S. Yanagida, J. Phys. Chem. B., 2001, 105, 1280 CrossRef CAS.
- H. Matsumoto and T. Matsuda., Electrochemistry, 2002, 70, 190 Search PubMed.
- R. Kawano and M. Watanabe, Chem. Commun., 2003, 330 RSC.
- C. M. Gordon, J. D. Holbrey, A. R. Kennedy and K. R. Seddon, J. Mater. Chem., 1998, 8, 2627 RSC.
- A. E. Bradley, C. Hardacre, J. D. Holbrey and M. Nieuwenhuyzen., Chem. Mater., 2002, 14, 629 CrossRef CAS.
- S. Nakade, S. Kambe, T. Kitamura, Y. Wada and S. Yanagida, J. Phys. Chem. B., 2001, 105, 9150 CrossRef CAS.
- Japanese Industrial Standard Committee, Japanese Industrial Standard, Solar simulators for amorphous solar cells and modules, JIS C 8933, Japanese Standards Association, Tokyo, 1995 Search PubMed.
- F. Pichot and B. A. Gregg, J. Phys. Chem. B., 2000, 104, 6 CrossRef CAS.
- W. Kubo, S. Kambe, S. Nakade, T. Kitamura, Y. Wada and S. Yanagida, J. Phys. Chem. B., 2003, 107, 4374 CrossRef CAS.
- M. Yoshio, T. Mukai, K. Kanie, M. Yoshizawa, H. Ohno and T. Kato, Adv. Mater., 2002, 14, 35 CrossRef CAS.
- A. B. McEwen, H. L. Ngo, K. LeCompte and J. L. Goldman, J. Electrochem. Soc., 1999, 146, 1687 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
