Discotic liquid crystals stabilized by interionic interactions: imidazolium ion-anchored paraffinic triphenylene†
Jin
Motoyanagi
a,
Takanori
Fukushima
*b and
Takuzo
Aida
*a
aDepartment of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8656, Japan. E-mail: aida@macro.t.u-tokyo.ac.jp; Fax: +81-3-5841-7310; Tel: +81-3-5841-7251
bAida Nanospace Project, Exploratory Research for Advanced Technology (ERATO), Japan Science and Technology Agency (JST), National Museum of Emerging Science and Innovation, 2-41 Aomi, Koto-ku, Tokyo, 135-0064, Japan. E-mail: fukushima@nanospace.miraikan.jst.go.jp; Fax: +81-3-3570-9183; Tel: +81-3-3570-9182
First published on 26th November 2004
Abstract
Incorporation of imidazolium ion functionalities into the paraffinic side-chain termini of a triphenylene derivative resulted in stabilization of a columnar mesophase of its liquid crystalline assembly, which was retained over a wider temperature range down to 4 °C by an externally added imidazolium-based ionic liquid.
Discotic liquid crystalline materials1 based on polyaromatic molecules have attracted increasing attention, because of their one-dimensional charge and energy transport capabilities in mesophases.2 Along with their high processabilities, they are potential components for organic electronic and optoelectronic devices.3 From this point of view, development of discotic liquid crystals having a broad mesophase range4 down to room temperature4e–g is an important subject. However, such thermotropic liquid crystals generally possess high crystal-to-mesophase transition temperatures. Furthermore, attempts to lower the crystallisation temperature usually result in lowering the clearing temperature.5 Here we report an interesting finding that interionic interactions among imidazolium salts can stabilize the columnar mesophase of a triphenylene (TP)-based discotic liquid crystal.
As exemplified by 1-alkyl-3-methylimidazolium tetrafluoroborates,6 some of these salts are liquids over a wide temperature range, even at cryogenic temperatures. Such ionic molten states are quite interesting, since interionic interactions involved usually facilitate crystallisation of electrolytes. Our molecular design is based on a TP derivative with paraffinic alkyl chains, whose termini are covalently attached to 1-methylimidazolium tetrafluoroborate (BF4)
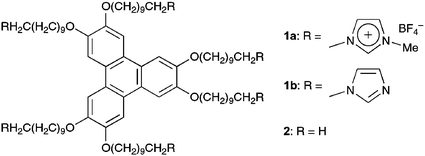
(1a). We expected that the imidazolium ion termini could prevent crystallisation of the alkyl chains, while interionic interactions7 among them may stabilize the columnar assembly of the TP moiety.
Compound 1a8 was obtained as a colourless waxy solid by N-alkylation of 1-methylimidazole with hexakis(10-bromodecyloxy)triphenylene, followed by anion exchange from Br− to BF4− using AgBF4. We found that this new class of TP derivative, in comparison with the parent paraffinic triphenylene (2),5 forms an enantiotropic liquid crystal with a wide LC temperature range. As shown in Fig. 1(a), polarized optical microscopy (POM) of 1a at 103 °C under crossed polarizing conditions displayed a fan texture characteristic of a columnar mesophase. Differential scanning calorimetry (DSC) of 1a showed phase transitions, where the mesophase was observed at 111–47 °C on cooling (Fig. 2(a)). In contrast, the paraffinic TP (2) without imidazolium ion functionalities has been reported to form a columnar mesophase only in a much narrower temperature range (69–58 °C, cooling run).5 We also found that an imidazole-anchored TP (1b),8 as a non-ionic reference, does not form any thermotropic mesophases, but gives a crystalline material that melts at 34 °C.8 On the other hand, when an imidazolium molten salt such as 1-hexyl-3-methylimidazolium tetrafluoroborate ([C6mim][BF4]) was added to 2, a macroscopic phase separation resulted. Thus, the columnar LC mesophase is retained over a wide temperature range by the imidazolium ion groups covalently incorporated into the paraffinic TP (2).
![Optical textures of (a)
1a at 103 °C, (b)
1a at 25 °C and (c) an equimolar mixture of 1a and [C6mim][BF4] at 25 °C, observed under crossed polarizing conditions of a microscope on cooling from the isotropic liquids at a rate of 1 °C min−1.](/image/article/2005/CC/b414649b/b414649b-f1.gif) | ||
| Fig. 1 Optical textures of (a) 1a at 103 °C, (b) 1a at 25 °C and (c) an equimolar mixture of 1a and [C6mim][BF4] at 25 °C, observed under crossed polarizing conditions of a microscope on cooling from the isotropic liquids at a rate of 1 °C min−1. | ||
![DSC traces on second heating/cooling cycle of (a)
1a and (b) an equimolar mixture of 1a and [C6mim][BF4]
(scan rate 10 °C min−1). G, glassy state; M, mesophase; DCol, discotic columnar mesophase; Iso, isotropic liquid.](/image/article/2005/CC/b414649b/b414649b-f2.gif) | ||
| Fig. 2 DSC traces on second heating/cooling cycle of (a) 1a and (b) an equimolar mixture of 1a and [C6mim][BF4] (scan rate 10 °C min−1). G, glassy state; M, mesophase; DCol, discotic columnar mesophase; Iso, isotropic liquid. | ||
Unexpectedly, the LC mesophase of 1a on cooling underwent a phase transition to an amorphous, viscous fluid (M in Fig. 2(a)), where the fan texture of 1a in POM disappeared completely (Fig. 1(b)). This behaviour is in contrast with those of ordinary LCs including 2, which are usually crystallised or transformed to glassy solids on cooling. We assume that this phenomenon is due to a possible competition of the π-stacking interaction among the TP units with a π-cation–π interaction9 between the anchored-imidazolium groups and the TP units, considering that the latter interaction is likely pronounced at lower temperatures. However, we found that 1a, upon mixing with an equimolar amount of [C6mim][BF4], forms a stable columnar LC phase, which is held even on cooling down to 4 °C without any phase separation. POM of the resultant mixture clearly displayed a columnar LC-originating fan texture (Fig. 1(c)). Furthermore, DSC of the mixture, on cooling from its isotropic liquid, showed the appearance of the LC phase at a higher temperature (117 °C) than in the absence of [C6mim][BF4] (111 °C) (Fig. 2(b)). Thus, the externally added molten salt significantly expands the mesophase temperature range of 1a from 47–111 to 4–117 °C (Fig. 2). We tentatively think that the imidazolium salt functionalities of 1a in the LC mesophase form a pseudo network by their interionic interaction, which stabilizes the columnar assembly to a certain extent. When an appropriate amount of [C6mim][BF4] is added to the system, it presumably participates in and reinforces the interionic network, so that the columnar mesophase is retained even at 4 °C.
In the phase diagram of 1a in the presence of [C6mim][BF4], the mesophase was observed at the mole fractions of [C6mim][BF4] ranging from 0.3 to 0.8 (Fig. 3). On the other hand, when the mole fraction of [C6mim][BF4] was out of the above range, dissolution of 1a (> 0.8) or no stabilization of the LC phase (< 0.3) took place. Although the mesophase of 1a, in the absence of [C6mim][BF4], displayed only weak X-ray diffraction (XRD) peaks, the combination of [C6mim][BF4] with 1a resulted in a notable enhancement of the diffraction intensity. An example is shown by XRD of a 0.2 ∶ 0.8 mixture of 1a and [C6mim][BF4] (Fig. 4), where diffraction peaks with d spacings of 15.4 and 7.75 Å were observed, along with a negligibly small alkyl halo at 4.5 Å. The XRD pattern is assignable to a hexagonal columnar mesophase with a lateral core-to-core separation of 17.8 Å, which is in good agreement with the diameter of 1a with tightly folded side chains (19 Å).
![Phase diagram of 1a in the presence of [C6mim][BF4], as determined by DSC on second heating runs. G, glassy state; M, mesophase; DCol, discotic columnar mesophase; Iso, isotropic liquid.](/image/article/2005/CC/b414649b/b414649b-f3.gif) | ||
| Fig. 3 Phase diagram of 1a in the presence of [C6mim][BF4], as determined by DSC on second heating runs. G, glassy state; M, mesophase; DCol, discotic columnar mesophase; Iso, isotropic liquid. | ||
![XRD pattern (scan rate of 4° min−1) of a mixture of 1a and [C6mim][BF4]
(0.2 ∶ 0.8), measured after an isotropic liquid was allowed to cool to 25 °C. Inset: magnified (× 100) diffraction pattern.](/image/article/2005/CC/b414649b/b414649b-f4.gif) | ||
| Fig. 4 XRD pattern (scan rate of 4° min−1) of a mixture of 1a and [C6mim][BF4] (0.2 ∶ 0.8), measured after an isotropic liquid was allowed to cool to 25 °C. Inset: magnified (× 100) diffraction pattern. | ||
In conclusion, we have developed a new triphenylene that forms a columnar LC mesophase over a wide temperature range from 47 to 111 °C, by incorporation of imidazolium salt-anchored long C10 side chains into the mesogenic core. With an externally added imidazolium molten salt, the columnar mesophase is maintained over a much wider temperature range from 4 to 117 °C. This finding is not only important for a new possibility of organic molten salts in materials science,9,10 but may also contribute to the development of novel anisotropic soft materials for directional ion conductivity11 and charge transport.
Notes and references
- (a) N. Boden and B. Movaghar, Handbook of Liquid Crystals, vol. 2B, ed. D. Demus, J. W. Goodby, G. W. Gray, H. W. Spiess and V. Vill, VCH, Weinheim, 1998, p. 781 Search PubMed; (b) M. D. Watson, A. Fechtenkötter and K. Müllen, Chem. Rev., 2001, 101, 1267 CrossRef CAS.
- (a) N. Boden, R. J. Bushby, J. Clements, B. Movaghar, K. J. Donovan and T. Kreouzis, Phys. Rev., 1995, 52, 13274 Search PubMed; (b) T. Kreouzis, K. J. Donovan, N. Boden, R. J. Bushby, O. R. Lozman and Q. Liu, J. Chem. Phys., 2001, 114, 1797 CrossRef; (c) M. O'Neill and S. M. Kelly, Adv. Mater., 2003, 15, 1135 CrossRef.
- (a) N. Boden, R. J. Bushby, J. Clements and B. Movaghar, J. Mater. Chem., 1999, 9, 2081 RSC; (b) C. D. Simpson, J. Wu, M. D. Watson and K. Müllen, J. Mater. Chem., 2004, 14, 494 RSC.
- (a) C. M. Paleos and D. Tsiourvas, Angew. Chem., Int. Ed. Engl., 1995, 34, 1696 CrossRef CAS; (b) E. O. Arikainen, N. Boden, R. J. Bushby, O. R. Lozman, J. G. Vinter and A. Wood, Angew. Chem., Int. Ed., 2000, 39, 2333 CrossRef; (c) T.-Q. Nguyen, R. Martel, P. Avouris, M. L. Bushey, L. Brus and C. Nuckolls, J. Am. Chem. Soc., 2004, 126, 5234 CrossRef CAS; (d) H. Bengs, M. Ebert, O. Karthaus, B. Kohne, K. Praefcke, H. Ringsdorf, J. H. Wendorff and R. Wüstefeld, Adv. Mater., 1990, 2, 141 CrossRef CAS; (e) C.-y. Liu, A. Fechtenkötter, M. D. Watson, K. Müllen and A. J. Bard, Chem. Mater., 2003, 15, 124 CrossRef CAS; (f) S. Kumar, D. S. S. Rao and S. K. Prasad, J. Mater. Chem., 1999, 9, 2751 RSC; (g) R. J. Bushby, N. Boden, C. A. Kilner, O. R. Lozman, Z. Lu, Q. Liu and M. A. Thornton-Pett, J. Mater. Chem., 2003, 13, 470 RSC.
- For example, see: C. Destrade, N. H. Tinh, H. Gasparoux, J. Malthete and A. M. Levelut, Mol. Cryst. Liq. Cryst., 1981, 71, 111 Search PubMed.
- (a) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef; (b) J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156 RSC; (c) J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC.
- J. Fuller, R. T. Carlin, H. C. De Long and D. Haworth, J. Chem. Soc., Chem. Commun., 1994, 299 RSC.
- See Supporting Information.
- T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300, 2072 CrossRef CAS.
- E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012 CrossRef CAS.
- M. Yoshio, T. Mukai, K. Kanie, M. Yoshizawa, H. Ohno and T. Kato, Adv. Mater., 2002, 14, 351 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: procedures for synthesis and characterization of 1a and 1b. DSC trace of 1b. See http://www.rsc.org/suppdata/cc/b4/b414649b/ |
| This journal is © The Royal Society of Chemistry 2005 |