[MoO2Cl2] as catalyst for hydrosilylation of aldehydes and ketones†
Ana C. Fernandes, Ricardo Fernandes, Carlos C. Romão and Beatriz Royo*
Instituto de Tecnologia Química e Biológica da Universidade Nova de Lisboa, Quinta do Marquês, EAN, Apt. 127, 2781-901 Oeiras, Portugal. E-mail: broyo@itqb.unl.pt; Fax: +351-214411277; Tel: +351-214469731
First published on 2nd December 2004
Abstract
The high valent molybdenum-dioxo complex [MoO2Cl2] catalyzes the addition of dimethylphenylsilane to aldehydes and ketones to afford the corresponding dimethylphenylsilyl ethers in quantitative yield.
The activation of a Si–H bond is one of the key steps in hydrosilylation and other catalytic reactions.1 Numerous transition metal complexes activate Si–H bonds via oxidative addition to form hydrido silyl species.2 Recently, Toste and coworkers have proposed an alternative mechanism for the catalytic hydrosilylation of carbonyl groups, in which a metal hydride species is produced by a [2+2]-type addition of the Si–H bond to a metal–oxygen π-bond (see Scheme 1).3 Similar σ-bond activations have been performed by M
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and M
S and M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N multiple bonds (M = Ti, Zr).4 In order to further explore the use of metal–oxo complexes as catalysts for the reduction of carbonyl groups, we studied the catalytic activity of the dioxomolybdenum compound [MoO2Cl2]
(1) in the hydrosilylation of aldehydes and ketones.‡ So far, the cis-MoO2 fragment has been the subject of extensive studies related to oxidation and oxygen transfer reactions but its catalytic activity in a reducing reaction has never been studied.5
N multiple bonds (M = Ti, Zr).4 In order to further explore the use of metal–oxo complexes as catalysts for the reduction of carbonyl groups, we studied the catalytic activity of the dioxomolybdenum compound [MoO2Cl2]
(1) in the hydrosilylation of aldehydes and ketones.‡ So far, the cis-MoO2 fragment has been the subject of extensive studies related to oxidation and oxygen transfer reactions but its catalytic activity in a reducing reaction has never been studied.5![[2+2]-Addition of a Si–H bond to a metal–oxo multiple bond.](/image/article/2005/CC/b414145h/b414145h-s1.gif) | ||
| Scheme 1 [2+2]-Addition of a Si–H bond to a metal–oxo multiple bond. | ||
We found that 1 is a highly effective catalyst for the hydrosilylation of aromatic aldehydes and also ketones (see eqn. 1 and 2).
 | (1) |
 | (2) |
The catalytic activity of 1 was tested using 4-nitrobenzaldehyde and dimethylphenylsilane with a reaction ratio of 1 ∶ 1.2 in the presence of 5 mol% of catalyst. The reaction was carried out at 25 °C in dichloromethane over 4 h to yield the corresponding silyl ether, dimethyl(4-nitrobenzyloxy)phenylsilane, in 96% yield. The formation of the silylated compound proceeds without by-products detectable by NMR spectroscopy.
Using the above catalytic conditions, we then examined the scope of MoO2Cl2-mediated hydrosilylation with a variety of aldehydes and ketones (see Table 1). As shown in Table 1, the catalytic reaction is suitable for a wide scope of aromatic aldehyde substrates, affording the desired silyl ether in quantitative yields. In the case of the 4-bromobenzaldehyde, the corresponding silyl ether was obtained accompanied by a substantial quantity of desilylated alcohol. Benzaldehyde derivatives containing functional groups were well tolerated, even though the yields isolated for the ester and cyano functionalities were lower, 67% and 32% respectively. Ketones are also converted into the silylated compounds although longer times and higher temperatures are required.
| Substrate | Reaction conditions | Productb | Yieldc |
|---|---|---|---|
| a All reactions were carried out in dichloromethane with 1.0 equiv. of aldehyde or ketone, 1.2 equiv. of PhMe2SiH, using 5 mol% of [MoO2Cl2].b Fully characterized by IR and NMR data.c Isolated, purified material.d Accompanied by 38% desilylated alcohol.e 75 ∶ 25 trans ∶ cis. | |||
| a | 25 °C/4 h | 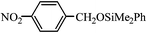 | 96% |
| b | 25 °C/4 h |  | 61%d |
| c | 25 °C/4 h |  | 97% |
| d | 25 °C/16 h |  | 32% |
| e | 25 °C/16 h |  | 67% |
| f | 40 °C/16 h |  | 68%e |
| g | 40 °C/16 h |  | 76% |
The catalytic reaction was found to work with triethylsilane but the yields of the corresponding ethyl ethers were significantly reduced (24% yield for (4-nitrobenzyloxy)triethylsilane and 18% yield for (4-bromobenzyloxy)triethylsilane after being stirred for 10 h at room temperature in dichloromethane). No catalysis was observed with the more sterically encumbered triphenylsilane. Replacement of dichloromethane by toluene as solvent afforded similar results.
When the catalytic reaction was monitored by 1H NMR no intermediates in the reaction were observed. The reaction of 1 with a stoichiometric amount of dimethylphenylsilane gave an intractable blue residue, probably due to decomposition of an intermediate hydride species formed by a [2+2]-addition of the Si–H bond to the Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The [2+2]-type addition of a halosilane (R3SiX) to a molybdenum–oxygen π-bond has been reported in the literature.6
O bond. The [2+2]-type addition of a halosilane (R3SiX) to a molybdenum–oxygen π-bond has been reported in the literature.6
In conclusion, we have demonstrated that the high oxidation state molybdenum complex [MoO2Cl2] is a highly effective catalyst for representative hydrosilylations of aldehydes and ketones with dimethylphenylsilane. Studies related to the addition of other σ-bonds to a Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit are being pursued.
O unit are being pursued.
This research was financially supported by FCT through project POCTI 37726/QUI/01.
Notes and references
- G. W. Parshall, S. D. Itell, Homogeneous Catalysts: The Applications and Chemistry of Catalysis by Soluble Transition Metal Complexes, Wiley, New York, 1992 Search PubMed.
- J. Y. Corey and J. Braddock-Wilking, Chem. Rev., 1999, 99, 175 CrossRef CAS.
- J. J. Kennedy-Smith, K. A. Nolin, H. P. Gunterman and F. D. Toste, J. Am. Chem. Soc., 2003, 125, 4056 CrossRef CAS.
- H. M. Hoyt, F. E. Michael and R. G. Bergman, J. Am. Chem. Soc., 2004, 126, 1018 CrossRef CAS; J. L. Polse, R. A. Andersen and R. G. Bergman, J. Am. Chem. Soc., 1998, 120, 13405 CrossRef CAS; Z. K. Sweeney, J. L. Polse, R. A. Andersen and R. G. Bergman, J. Am. Chem. Soc., 1998, 120, 7825 CrossRef CAS; Z. K. Sweeney, J. L. Polse, R. A. Andersen, R. G. Bergman and M. G. Kubinec, J. Am. Chem. Soc., 1997, 119, 4543 CrossRef CAS.
- F. E. Kühn, M. Groarke, È. Bencze, E. Herdtweck, A. Prazeres, A. M. Santos, M. J. Calhorda, C. C. Romão, I. S. Gonçalves, A. D. Lopes and M. Pillinger, Chem. Eur. J., 2002, 8, 2370 CrossRef CAS; R. H. Holm, Chem. Rev., 1987, 87, 1401 CrossRef CAS.
- H. Arzoumanian, H. Krentzien, C. Corao, R. Lopez and G. Agrifoglio, Polyhedron, 1995, 14, 2887 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR and IR data. See http://www.rsc.org/suppdata/cc/b4/b414145h/ |
| ‡ Typical experimental procedure for the hydrosilylation of aldehydes and ketones with dimethylphenylsilane: All operations were carried out under nitrogen. In a small flask, a mixture of aldehyde or ketone (1.01 mmol), PhMe2SiH (1.20 mmol), and a catalytic amount of 1 (0.050 mmol) was dissolved in dichloromethane (5 mL). The mixture was stirred (the reaction time and the temperature are indicated in Table 1) and monitored periodically by TLC. Upon completion, the reaction mixture was diluted with hexane, loaded directly on to a silica gel column and chromatographed with the appropriate mixture of hexane and diethyl ether to give the silyl ether. |
| This journal is © The Royal Society of Chemistry 2005 |

