Stereoselective hydrogenations of aryl-substituted dienes
Xiuhua
Cui
,
James W.
Ogle
and
Kevin
Burgess
*
Department of Chemistry, Texas A&M University, Box 30012, College Station, TX 77842-3012, USA. E-mail: burgess@tamu.edu; Fax: +1 979 845 8839; Tel: +1 979 845 4345
First published on 6th January 2005
Abstract
The carbene complex 1 can mediate hydrogenations of dienes with up to 20∶1.0 diastereoselectivity and 99% ee; the scope and limitations of these reactions were investigated.
Asymmetric hydrogenations of dienes have the potential to generate two or more chiral centers with controlled relative and absolute stereochemistries. This is exciting with respect to synthetic applications, but challenging for catalyst design and optimization of conditions. Little is known about asymmetric hydrogenations of dienes of any kind.1–4 Kinetic studies by us elucidated aspects of the pathways followed for asymmetric hydrogenation of 2,3-diphenylbutadiene mediated by the carbene oxazoline complex 1,1,5,6 but the diastereoselectivity in favor of the DL-product was low, and the maximum enantiomeric excesses obtained for that substrate were in the high 80's (Table 1, entry 1). Here we report that higher stereoselectivities can be achieved for other categories of dienes. Further, the yields and conversion obtained for these hydrogenations using catalyst 1 are higher than for the archetypical achiral iridium catalyst.
| Entry | Diene | Conv.a (%) | Yielda (%) | ent∶mesoa | eea (%) |
|---|---|---|---|---|---|
| a Determined by GC using a β- or a γ-CD column;7 absolute stereochemistries were not determined except for entry 1. b MePh as solvent. c 1 atm H2, 24 h. d Tetrasubstituted monoene as the major by-product. e 10 atm, 2 h. f ClCH2CH2Cl as solvent. g 2 mol% catalyst. | |||||
| type 1 | |||||
| 1bc |

|
100 | 96d | 1.0∶2.9 | 87 |
| 2e |

|
100 | 100 | 2.1∶1.0 | 86 |
| 3be |
|
88 | 65 | 1.0∶2.9 | 24 |
| type 2 | |||||

|
|||||
| 4f |

|
100 | 69d | 1.3∶1.0 | 98 |
| 5f |

|
100 | 67d | 1.3∶1.0 | 97 |
| 6 |

|
100 | 96 | 1.8∶1.0 | 99 |
| type 3 | |||||

|
|||||
| 7g |

|
93 | 92 | 14∶1.0 | 98 |
| 8g |

|
100 | 100 | 20∶1.0 | 99 |
| 9g |

|
100 | 100 | 1.2∶1.0 | 70 |
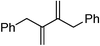
Table 1 summarizes results for asymmetric hydrogenations of some aryl-substituted dienes, beginning with ones containing 1,1-disubstituted alkene fragments (type 1).† 1,1-Disubstituted alkenes are difficult to hydrogenate with high enantioselectivities.5,6,8 Consequently, it seemed likely that dienes containing 1,1-disubstituted alkene fragments also might be difficult to hydrogenate with high enantioselectivities; this was in fact the case. Entries 2 and 3 feature substrates that, like 2,3-diphenylbutadiene, contain 1,1-disubstituted alkene fragments. The 1,5-diene shown in entry 2 was reduced to the corresponding diaryl alkane isomers without contamination from partial reduction and/or double bond migration products. The diastereoselectivity in favor of the ent-form was greater than for 2,3-diphenylbutadiene, and the enantioselectivity of that major product was about the same. However, the corresponding reduction of the 1,3-diene in entry 3 gave a poor yield of the product and inferior stereoselectivities.
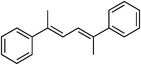
Entries 4–6 of Table 1 feature (E,E)-1,4-diaryl-2,3-dimethylbuta-1,3-dienes (type 2). The benzenoid systems give quantitative conversions of the starting material, but moderate yields of the product. The mass balance is largely accounted for by formation of the double bond migration products 2. Curiously, hydrogenation of the furan-substituted system in entry 6 is not complicated in the same way. All three substrates of this class are marginally selective for the ent-products, and these formed in excellent enantiomeric excesses.
(E,E)-1,4-Diaryl-1,4-dimethyl-1,3-dienes tend to be the best-behaved substrates in the series of dienes examined (entries 7–9). The benzenoid systems give high ent/meso ratios, and the optically active product forms in high ee's. However, the diene with two furan groups is again anomalous (entry 9) insofar as both aspects of the stereoselectivity were less.
Collectively, the data in Table 1 indicate several trends. Dienes with 1,1-disubstituted alkene functionalities can be hydrogenated with moderate enantioselectivities, though there are some unpredictable variations with molecular structure. (E,E)-1,4-Diaryl-2,3-dimethylbuta-1,3-diene substrates tend to be better behaved, and give higher enantioselectivities, though double bond migration to unreactive tetrasubstituted alkenes can reduce the yields. However, the more hindered o-tolyl system 3 was a poor substrate only giving 8% yield of the reduction product under the same conditions. Side products from migration reactions were not observed for the furan-substituted system in entry 6, unlike the benzenoid analogs in entries 4 and 5, and that may be indicative of a coordinating effect and/or the enhanced nucleophilicity of the π-bonds. Those same parameters may be the origin of the relatively poor enantioselectivity observed for the furan-substituted system in entry 9 relative to the other (E,E)-1,4-dimethyl-1,3-dienes. Related to this, another observation provides circumstantial evidence that coordination effects are important. Thus, the analogous thiophene-substituted alkene 4 was a poor substrate giving only 20% conversion (de/ee not determined).
Crabtree's Ir(py)(PCy3)(COD)PF65 is the most widely recognized homogeneous catalyst for hydrogenations of unfunctionalized, highly hindered alkenes.9 A selection of dienes were also hydrogenated with Crabtree's catalyst, for comparison (Table 2), but only after some controls were performed. It was shown that the conversions were less when a 50 atm H2 pressure (as used in the asymmetric hydrogenations) was used. Moreover, since Pfaltz's10 and our groups5 have observed that the BARF counter-ion has a beneficial influence on conversions in these Ir-mediated reactions, we performed experiments with Crabtree's catalyst in the presence of 5 mol% of NaBARF; however, the outcome was marginally worse (data not shown).
Table 2 shows that the conversions with Crabtree's catalyst tend to be less than those observed in the corresponding asymmetric reactions (Table 1). Moreover, the yields tend to be significantly lower than the conversions reflecting decomposition of Crabtree's catalyst before the reactions were complete, leading to partial reduction products. Catalyst 1 is more robust in the hydrogenations of these substrates. None of the ent/meso selectivities for Crabtree's catalyst are high, and all but one of the reactions favored the meso product.
This is a comprehensive account of all the asymmetric hydrogenations of aryl-substituted dienes that we have studied so far. Even substrates that gave relatively poor results were mentioned, to give a balanced impression of the scope and limitations of the reaction. Good ee's can be obtained for two of the three substrate types studied, and excellent ent/meso diastereoselectivities were obtained for the type 3 dienes. The conversions were high, much better than for Crabtree's catalyst under similar conditions, though some material was diverted to the relatively unreactive tetrasubstituted alkenes 2 in the particular case of the type 2 alkenes. The next step in this project will be to investigate hydrogenations of dienes and trienes that give more useful chirons for natural product syntheses.
Financial support for this work was provided by The Robert Welch Foundation. We thank Dr Shane Stichy and the TAMU/LBMS-Applications Laboratory for MS support, and NMR Laboratory at Texas A&M University, supported by a grant from the National Science Foundation (DBI-9970232) and the Texas A&M University System.
Notes and references
- X. Cui and K. Burgess, J. Am. Chem. Soc., 2003, 125, 14212 CrossRef CAS.
- H. Muramatsu, H. Kawano, Y. Ishii, M. Saburi and Y. Uchida, J. Chem. Soc., Chem. Commun., 1989, 769 RSC.
- M. J. Burk, J. G. Allen and W. F. Kiesman, J. Am. Chem. Soc., 1998, 120, 657 CrossRef CAS.
- V. Beghetto, U. Matteoli and A. Serivanti, J. Chem. Soc., Chem. Commun., 2000, 155 RSC.
- M. C. Perry, X. Cui, M. T. Powell, D.-R. Hou, J. H. Reibenspies and K. Burgess, J. Am. Chem. Soc., 2003, 125, 113 CrossRef CAS.
- K. Burgess, M. C. Perry and X. Cui, in New Methodologies in Asymmetric Catalysis, ed. S. Malhotra, ACS Publications, Washington, DC, 2003 Search PubMed.
- A. Shitangkoon and G. Vigh, J. Chromatogr. A., 1996, 738, 31 CrossRef CAS.
- A. Pfaltz, J. Blankenstein, R. Hilgraf, E. Hormann, S. McIntyre, F. Menges, M. Schonleber, S. P. Smidt, B. Wustenberg and N. Zimmermann, Adv. Synth. Catal., 2003, 345, 33 CrossRef CAS.
- R. Crabtree, Acc. Chem. Res., 1979, 12, 331 CrossRef CAS.
- D. Drago, P. S. Pregosin and A. Pfaltz, Chem. Commun., 2002, 286 RSC.
Footnote |
| † High pressure hydrogenations were performed using a Parr Bomb. A test tube (∼1.0 × 10 cm2) containing diene substrate (0.1 or 0.2 mmol), iridium catalyst 1 or 5, solvent and a spin bar was placed in the bomb. The bomb was flushed with hydrogen for 1 min without stirring. The mixture was then stirred at 700 rpm at the desired pressure for the designated time, the bomb was then vented and the solvent evaporated. The crude product was passed through a silica plug (hexanes–ethyl acetate = 7∶3). The conversion, yield, ee and ent/meso ratio were then determined by GC with a β- or a γ-CD column.7 |
| This journal is © The Royal Society of Chemistry 2005 |