Activation of a 1,10-phenanthroline ligand on a rhenium tricarbonyl complex†
Luciano
Cuesta
a,
Eva
Hevia
a,
Dolores
Morales
a,
Julio
Pérez
*a,
Víctor
Riera
a,
Elena
Rodríguez
a and
Daniel
Miguel
b
aDepartamento de Química Orgánica e Inorgánica-IUQOEM, Facultad de Química-CSIC, Universidad de Oviedo, 33006, Oviedo, Spain. E-mail: japm@fq.uniovi.es; Fax: 34 98510 3446; Tel: 34 98510 3467
bDepartamento de Química Inorgánica, Facultad de Ciencias, Universidad de Valladolid, 47071, Valladolid, Spain
First published on 22nd November 2004
Abstract
The reactions of complex [Re(PPh2)(CO)3(phen)] with either methyl acrylate or methyl propiolate afford products in which the phen ligand has been activated.
2,2′-Bipyridine (bipy), 1,10-phenanthroline (phen) and their substituted analogues form robust metal complexes that have played important roles in several areas of chemistry.1–3 Coordinated bipy and phen are so inert that the nucleophilic attack of hydroxide anion on a N-adjacent carbon, proposed to explain the anomalous behaviour of some bipy or phen complexes,4 is discussed in textbooks5 and continues to attract interest while remaining controversial.6,7
We have recently found that complexes [MoY(η3-allyl)(CO)2(N–N)] and [ReY(CO)3(N–N)] (Y = alkoxo, hydroxo or amido; N–N = bipy or phen) react with dimethylacetylene dicarboxylate (DMAD).8–11 The results are consistent with initial attack by the Y ligand at one of the acetylenic carbon atoms and addition of the putative resulting vinyl carbanion to either the metal (for M–OR complexes with displacement of oxygen) or a CO ligand (hydroxo or amido complexes). These results are depicted in Scheme 1.
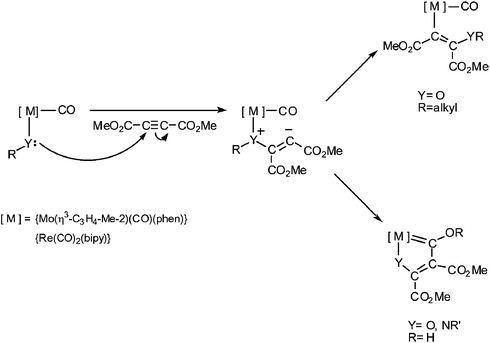 | ||
| Scheme 1 | ||
We also reported the synthesis and highly nucleophilic character of the phosphido complex [Re(PPh2)(CO)3(phen)] (1).12 Studies of the reactivity of terminal phosphido complexes are scarce.13–16 Here we report that 1 reacts with electron-poor olefinic or acetylenic substrates to afford products resulting from addition to the N-adjacent carbon of phen.
The phosphido complex 1 was found to react with methyl acrylate and with methyl propiolate, affording the new compounds 2 and 3, respectively, as the single products (see Scheme 2).17,18 These products could be isolated in high yield and characterized both in solution by IR and NMR (1H, 13C and 31P) spectroscopies and in solid state by single-crystal X-ray diffraction. The IR spectra indicated the persistence of fac-Re(CO)3 moieties in 2 and 3, thus ruling out intramolecular attack on one of the carbonyl ligands, as have been found in the reactions of hydroxo and amido complexes with DMAD (see above) and in previous studies of the reactivity of iron, molybdenum and tungsten phosphido complexes. The observation of coupling to phosphorus in the signals of the carbonyl ligands in the 13C NMR spectra strongly suggests that the Re–P bond also remains intact. The 1H NMR spectra revealed the desymmetrization of the phenanthroline ligand. These features are consistent with the solid-state structures, showed in Figs. 1 (compound 2) and 2 (compound 3).‡
 | ||
| Fig. 1 Thermal ellipsoid (30%) plot of 2. Selected bond lengths (Å): N(1)–C(11) 1.452(11), C(11)–C(12) 1.540(14), C(12)–C(13) 1.307(14). | ||
 | ||
| Scheme 2 | ||
The molecules of 2 and 3 are distorted octahedra consisting of fac-Re(CO)3 moieties bonded to the asymmetric PNN′ donor set of a new tridentate ligand in which the C2 unit from the olefin or acetylene links the phosphorus atom to the C2 atom of the phen backbone thus creating a six-membered metallacycle. The loss of aromaticity in the phen ring attacked resulted in its distortion from planarity. The phosphorus is bonded to the non-substituted atom of the olefin or acetylene, a fact consistent with the mechanistic proposal (in line with our previous studies on the reactivity of alkoxo, hydroxo and amido complexes) shown in Scheme 3, since the nucleophilic phosphido group would attack the more electrophilic carbon of the organic substrate. The bonding scheme depicted for the attacked phen ring of the product in Scheme 3 is substantiated by the comparison of the intraligand distances in 1 (in Å, numbering scheme as for 2 and 3: N(1)–C(11) 1.327(11), C(11)–C(12) 1.387(15), C(12)–C(13) 1.354(17)) with those found in 2 and 3 (see captions of Figs. 1 and 2).
 | ||
| Fig. 2 Thermal ellipsoid (30%) plot of 3. Selected bond lengths (Å): N(1)–C(11) 1.469(8), C(11)–C(12) 1.522(9), C(12)–C(13) 1.321(10). | ||
 | ||
| Scheme 3 | ||
In summary, we have presented evidence of two reactions resulting in the activation of a phenanthroline ligand under very mild conditions, a transformation without precedents in the chemistry of carbonyl complexes.19 Studies of the reactivity of 1 with other electrophiles are under way in our laboratory.
We thank Ministerio de Ciencia y Tecnología for support of this work (Projects BQU2003-08649 and BQU2002-03414).
Notes and references
- P. G. Sammes and G. Yahioglu, Chem. Soc. Rev., 1994, 327 RSC.
- C. Kaes, A. Katz and M. W. Hosseini, Chem. Rev., 2000, 100, 3553 CrossRef CAS.
- G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129 CrossRef CAS.
- R. D. Gillard, Coord. Chem. Rev., 1975, 16, 67 CrossRef CAS.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, John Wiley & Sons, Inc., New York, 6th edn., 1999, p. 351 Search PubMed.
- E. C. Constable, Metals and Ligand Reactivity, VCH, Weinheim, 1996, p. 245 Search PubMed.
- C. S. McInnes, B. R. Clare, W. R. Redmond, C. R. Clark and A. G. Blackman, Dalton Trans., 2003, 2215 RSC.
- E. Hevia, J. Pérez, L. Riera, V. Riera, I. del Río, S. García-Granda and D. Miguel, Chem. Eur. J., 2002, 8, 4510 CrossRef CAS.
- D. Morales, J. Pérez, L. Riera, V. Riera, D. Miguel, M. E. G. Mosquera and S. García-Granda, Chem. Eur. J., 2003, 9, 4132 CrossRef CAS.
- L. Cuesta, D. C. Gerbino, E. Hevia, D. Morales, M. E. Navarro Clemente, J. Pérez, L. Riera, V. Riera, D. Miguel, I. del Río and S. García-Granda, Chem. Eur. J., 2004, 10, 1765 CrossRef CAS.
- E. Hevia, J. Pérez, V. Riera and D. Miguel, Organometallics, 2003, 22, 257 CrossRef CAS.
- E. Hevia, J. Pérez, V. Riera and D. Miguel, Organometallics, 2002, 21, 1966 CrossRef CAS.
- (a) U. Segerer, S. Blaurock, J. Sieler and E. Hey-Hawkins, Organometallics, 1999, 18, 2838 CrossRef CAS; (b) U. Segerer, J. Sieler and E. Hey-Hawkins, Organometallics, 2000, 19, 2445 CrossRef CAS; (c) U. Segerer, S. Blaurock, J. Sieler and E. Hey-Hawkins, J. Organomet. Chem., 2002, 608, 21 CrossRef.
- M. T. Ashby and J. H. Enemark, Organometallic, 1987, 6, 1323 CrossRef CAS.
- H. Adams, N. A. Bailey, A. N. Day and M. J. Morris, J. Organomet. Chem., 1991, 407, 247 CrossRef CAS.
- H. Adams, N. A. Bailey, P. Blenkiron and M. J. Morris, J. Chem. Soc., Dalton Trans., 2000, 3074 RSC.
- Synthesis of 2: Methyl acrylate (11 µL, 0.13 mmol) was added to a solution of 1 (0.081 g, 0.13 mmol) in THF (10 mL). The colour of the solution immediately changed from blue to red. The solution was stirred for 20 h, in vacuo concentration and precipitation with hexane afforded a red microcrystalline solid. Slow evaporation of a concentrated solution of 2 in diethyl ether–CH2Cl2 afforded crystals of good quality for an X-ray determination. Yield: 0.079 g, 84%. Anal. Calc. for C31H24N2O5PRe: C, 51.59; H, 3.35; N, 3.88. Found: C, 51.42; H, 3.52; N, 3.93%. IR (THF): 2014, 1920, 1892 (νCO). 1H NMR (C6D6): δ 8.15 [m, 1H], 7.53 [m, 1H], 7.01–6.94 [m, 6H], 6.76–6.65 [m, 3H], 6.56 [m, 1H], 6.41 [m, 3H], 6.12 [dd (8.54, 5.13), 1H], 6.03 [d (7.97), 1H], 5.30 [m, 2H], 3.84 [m, 1H], 3.33 [s, 3H, Me], 2.97 [m, 1H], 2.78 [m, 1H]. 13C{1H} NMR (C6D6): δ 198.9 [t (9.44), ReCO], 191.9 [d (74.79) ReCO], 172.9 [d (12.84 Hz), CO2Me] 155.4, [s, phen′], 148.2, 146.7, 136.7, 135.9, 135.4, 134.2, 132.5, 131.7, 127.0, 125.8, 121.9 [phen′ and Ph], 115.6, 107.3 [s, C3, C4 phen′], 68.4 [s, C5 phen′], 52.1 [s, CO2CH3], 49.3 [s, PCH2C(H)CO2Me], 32.9 [d (27.95 Hz), PCH2]. 31P{1H} NMR (C6D6): δ −0.86.
- Synthesis of 3: Methyl propiolate (8 µL, 0.09 mmol) was added to a solution of 1 (0.050 g, 0.08 mmol) in THF (10 mL) causing a colour change from blue to red. The mixture was stirred for 5 min and a microcrystalline solid was obtained by addition of hexane. The solid was dissolved in CH2Cl2 and filtered through alumina (activation grade IV). The solution was concentrated in vacuo, layered with hexane and stored at −20 °C, affording purple crystals, one of which was used for the X-ray structure determination. Yield: 0.047 g, 81%. Anal. Calc. for C31H22N2O5PRe: C, 51.74; H, 3.08; N, 3.89. Found: C, 51.22; H, 3.02; N, 3.94%. IR (THF): 2015, 1922, 1896 (νCO). 1H NMR (C6D6): δ 8.13 [m, 1H], 7.27 [m, 1H], 7.00 [m, 4H], 6.87 [dt (8.54, 1.42), 1H], 6.72 [m, 2H], 6.60–6.57 [m, 2H], 6.47–6.42 [m, 3H], 6.25 [dd (9.68, 1.42), 1H], 6.03 [dd (8.25, 4.84), 1H], 5.09 [d (8.25) 1H], 5.51 [dd (9.68, 4.84), 1H], 3.27 [s, 3H, Me]. 13C{1H} NMR (CD2Cl2): δ 198.3 [d (6.5), ReCO], 196.9 [d (8.1), ReCO], 193.0 [d (75.09), ReCO], 167.5 [d (15.34), CO2Me], 156.7, 156.3, 147.6, 144.7, 136.6, 1 [s, phen′], 134.7, [d (12.1), C1], 132.6, [d (11.3), C2], 131.7, [s, phen′], 129.5 [d (7.3), Ph], 129.1 [d (10.4), Ph], 128.6, [s, phen′], 127.9 [d (9.7 Hz), Ph], 127.8 [s, Ph], 127.3, 121.9, 119.8, [s, phen′], 114.3, 107.7 [s, C3, C4 phen′], 64.7 [s, C5 phen′], 52.6 [s, CO2CH3]. 31P{1H} NMR (C6D6): δ 3.41.
- (a) L. M. Kobriger, A. K. McMullen, P. E. Fanwick and I. P. Rothwell, Polyhedron, 1989, 8, 77 CrossRef CAS; (b) T. M. Cameron, J. C. Gordon, B. L. Scott and W. Tumas, Chem. Commun., 2004, 1398 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental details and atom-labeling scheme for phen′. See http://www.rsc.org/suppdata/cc/b4/b412447b/ |
| ‡ CCDC reference numbers 246293 (2) and 246294 (3). See http://www.rsc.org/suppdata/cc/b4/b412447b/ for crystallographic data in .cif or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |
