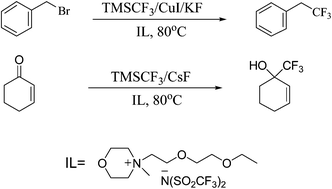
The new ionic liquids (5a–8a) were used as reaction media for nucleophilic trifluoromethylation reactions of trifluoromethyl(trimethyl)silane with (1) aryl, allyl, benzyl, and alkyl halides in Cu(I)-mediated C–C bond formation reactions, and (2) carbonyl functionalities catalyzed with Ph3P or CsF. In addition, conversion of benzyl bromide as a model compound to benzyl fluoride was examined in 6a using CsF as the fluorinating reagent. The morpholinium-based ionic liquid (6a) stood out as an efficient solvent system comparable to organic solvents and superior to the other new ionic liquids prepared in this work as well as to [bmim]+[PF6]−. Neat reactions of N-methyloxazolidine (1), N-methylmorpholine (2), N-methylimidazole (3) or N-methyltriazole (4) with 2-(2-ethoxyethoxy)ethyl bromide (BrCH2CH2OCH2CH2OCH2CH3, 9) or 2-bromoethyl methyl ether (BrCH2CH2OCH3, 10) at 75 or 105 °C gave the N-(2-ethoxyethoxy)ethyl- or N-methoxyethyl-substituted oxazolidinium, morpholinium, imidazolium and triazolium quaternary bromides (1a–4a, 1b–4b) which were metathesized with LiN(SO2CF3)2 to form the respective room-temperature liquid bis(trifluoromethanesulfonyl)amides 5a–8a and 5b–8b in high yields with transition or melting points <−78 °C as determined by DSC. All of the ionic liquids are thermally stable to >310 °C as determined by thermogravimetric analyses (TGA). Densities range between 1.29 and 1.53 g cm−3 at 25 °C.