Palladium–phosphinous acid-catalyzed Sonogashira cross-coupling reactions in water†
Christian
Wolf
* and
Rachel
Lerebours
Department of Chemistry, Georgetown University, Washington, DC 20057, USA. E-mail: cw27@georgetown.edu; Fax: (+1) 202 687 6209; Tel: (+1) 202 687 3468
First published on 30th June 2004
Abstract
A palladium–phosphinous acid-catalyzed Sonogashira cross-coupling reaction that proceeds in water under air atmosphere in the absence of organic co-solvents has been developed. Disubstituted alkynes have been prepared in up to 91% yield by POPd-catalyzed coupling of various aryl halides including chlorides in the presence of tetrabutylammonium bromide and pyrrolidine or NaOH.
Palladium-catalyzed Suzuki, Stille, Hiyama, Negishi, Buchwald–Hartwig, Kumada and Heck coupling reactions are among the most powerful carbon–carbon bond forming reactions.1 The recent development of highly active palladium complexes bearing bulky, electron-rich phosphane ligands has allowed chemists to extend these methods from reactive substrates such as aryl iodides, bromides, and triflates, to less reactive but inexpensive and readily available chlorides.2 Because of the instability of most catalysts and coupling reagents, cross-coupling reactions are usually carried out in polar organic solvents under inert and anhydrous conditions. However, from an environmental and economic standpoint, it is desirable to avoid any use of hazardous and expensive organic solvents. Water or aqueous solutions have been recognized as a very attractive medium for organic reactions because solvents do not have to be dried prior to use, and products can easily be isolated after completion of the reaction by extraction which greatly facilitates operation.3 Recent efforts to introduce water into cross-coupling reactions have resulted in aqueous Suzuki,4 Buchwald–Hartwig,5 Stille,6 Hiyama-like,7 and Heck8 reactions.9 Leadbeater recently described a microwave-assisted Suzuki coupling method using palladium acetate in water.10
Sonogashira cross-coupling of terminal alkynes with aryl and vinyl halides has attracted increasing attention. A variety of aryl halides including electron-deficient heteroaryl chlorides has been employed in palladium-catalyzed coupling reactions with terminal alkynes to afford versatile precursors for the formation of fused aromatic heterocycles, whereas the coupling of less reactive electron-rich aryl chlorides remains challenging.11 Typically, the Sonogashira reaction requires inert and anhydrous reaction conditions and only a few studies using water as an additive or co-solvent have been reported. Sub-stoichiometric amounts of water have been found to promote the formation of symmetrical and unsymmetrical bisarylethynylenes in a modified Sonogashira-type coupling reaction.12 The heterogeneous cross-coupling of aryl iodides and alkynes using Pd-catalysts immobilized on mesoporous silica under aqueous conditions has been reported to suffer from significant leaching.13 Kotschy and co-workers observed that catalyst leaching can be limited to less than 2% using Pd(0) immobilized on charcoal in CuI-promoted Sonogashira couplings of aryl halides in aqueous dimethyl acetamide.14 Palladium on carbon has successfully been used to catalyze the arylation of N-propargylated amino acids at terminal and nonterminal peptide positions in aqueous DMA.15 The employment of water-soluble palladium(II) acetate and sulfonated triphenylphosphine in a water–acetonitrile solution enabled Amatore and co-workers to conduct Sonogashira couplings of aryl iodides under homogeneous conditions.16 It is noteworthy that the presence of water has been found to be essential to the coupling reaction of aryl iodides and various alkynoates when using an amphiphilic polystyrene–poly(ethylene glycol) resin-supported palladium–phosphine complex.17 Dibowski and co-workers utilized palladium–guanidinophosphane catalysts for the coupling of water-soluble aryl iodides and terminal alkynes under mild and biocompatible aqueous conditions.18 Their procedure allowed the bioconjugation of peptide derivatives without compromising their native structure and biological activity. A similar strategy has recently been employed in the chemoselective coupling of aryl iodide-functionalized peptides to a trialkyne nucleus under both acidic and basic aqueous conditions.19
Recently, palladium–phosphinous acid complexes have been introduced to a variety of C–C bond forming reactions by us and others.20 We have reported the use of these electron-rich Pd-complexes in Heck, Stille, and Hiyama couplings and also in amination and thiation reactions of aryl chlorides and bromides.21 We also found that palladium–phosphinous acid complexes exhibit high stability to air and water and afford high yields in Stille coupling reactions in water.22 Herein, we describe the usefulness of palladium–phosphinous acid complexes [(t-Bu)2P(OH)]2PdCl2 (POPd), [[(t-Bu)2P(OH)(t-Bu)2PO)]PdCl]2 (POPd1) and [(t-Bu)2P(OH)PdCl2]2 (POPd2) in Sonogashira cross-coupling reactions of aryl halides in water (Fig. 1).
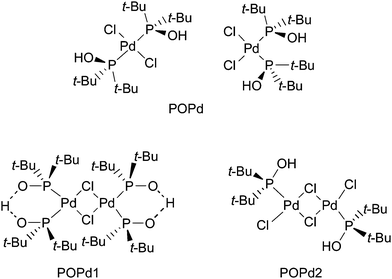 | ||
| Fig. 1 Structures of POPd, POPd1 and POPd2. | ||
To investigate the feasibility of aqueous Sonogashira coupling, we screened the catalytic performance of POPd, POPd1, and POPd2 in the cross-coupling reaction of 3-bromopyridine, 1, and phenylacetylene, 2, in the presence of pyrrolidine and tetrabutylammonium bromide, TBAB, in water under air without any organic co-solvents or surfactant additives (Scheme 1). We did not observe product formation using POPd1 and POPd2 but obtained alkyne 3 in 80% yield with POPd as the catalyst (Table 1, entries 1–3). Optimization studies revealed that the coupling reaction requires two equivalents of the terminal alkyne and does not proceed in the absence of TBAB or at lower temperature (Table 1, entries 4–6).
 | ||
| Scheme 1 Aqueous Sonogashira coupling of 3-bromopyridine, 1, and phenylacetylene, 2. | ||
| Entry | Catalyst | Base | Additives | Yield (%)a |
|---|---|---|---|---|
| a General reaction conditions: 100 mg of 1, 2 equivalents of 2, 10 mol% Pd catalyst, 1.0 equivalent of TBAB in 5 mL of deionized water at 135–140 °C for 5 h in a closed vessel. b No product formation was observed. c 1.5 equivalents of 2. d Reaction temperature was 100 °C. | ||||
| 1 | POPd | pyrrolidine | TBAB | 80 |
| 2 | POPd1 | pyrrolidine | TBAB | —b |
| 3 | POPd2 | pyrrolidine | TBAB | —b |
| 4 | POPd | pyrrolidine | TBAB | 60c |
| 5 | POPd | pyrrolidine | TBAB | —d |
| 6 | POPd | pyrrolidine | none | —b |
| 7 | POPd | Cy2NMe | TBAB | 10 |
| 8 | POPd | 2,2,6,6-TMP | TBAB | 10 |
| 9 | POPd | NaOAc | TBAB | 44 |
| 10 | POPd | Cs2CO3 | TBAB | 10 |
| 11 | POPd | pyrrolidine | TBAB/CuI | 90 |
| 12 | POPd | NaOH | TBAB/CuI | 81 |
We then decided to screen various organic and inorganic bases. In general, we obtained superior results with pyrrolidine. Replacement of pyrrolidine by N-methyldicyclohexylamine (Cy2NMe), 2,2,6,6-tetramethylpiperidine (TMP), sodium acetate or caesium carbonate resulted in significantly decreased yields of 3 (entries 7–10). Employing other additives than TBAB in the reaction mixture is not necessary albeit the yield of 3 further increased to 90% in the presence of 10 mol% of CuI (entry 11). Interestingly, the CuI-promoted POPd-catalyzed Sonogashira coupling of 1 and 2 affords 3 in remarkable 81% yield in 0.5 M sodium hydroxide solution (entry 12).
It is assumed that reduction and deprotonolysis of the palladium phosphinous acid complex results in the formation of a highly active anionic Pd(0) complex that undergoes oxidative addition with aryl halides in water (Scheme 2). Complexation by the terminal acetylene and subsequent deprotonation gives an intermediate complex that generates disubstituted alkynes via reductive elimination. Thus, sodium hydroxide or pyrrolidine fulfil two fundamental functions, i.e., activation of the catalyst for oxidative addition and alkyne deprotonation during catalysis.
 | ||
| Scheme 2 Proposed catalytic cycle of the POPd-catalyzed Sonogashira coupling. | ||
We applied our optimized coupling procedure to a variety of aryl halides and alkynes to determine the scope of the POPd-catalyzed Sonogashira reaction in water (Table 2). Aryl iodides and bromides were converted to the corresponding alkynes in 70–91% yield (entries 1–5, 9–11). It is noteworthy that nitrile and carbonyl functions are tolerated under the reaction conditions employed in this study. We obtained alkynes 9 and 20 from 2-bromobenzonitrile, 8, and 3-bromoacetophenone, 19, in 81% and 70% yield, respectively (entries 4 and 10). As expected, 3-chloropyridine, 16, affords lower yields than its bromo derivative 1 (entries 1 and 8). Introduction of ortho-substituents to phenylacetylene does not result in lower yields and we obtained alkyne 18 from 2-methylphenylacetylene, 17, and 1 in 90% (entry 9). As we had experienced with 3-bromopyridine, 1, replacement of pyrrolidine by NaOH slightly decreases the results of the Sonogashira coupling between phenylacetylene and bromonaphthalenes 4 and 6 (entries 2 and 3). The pyrrolidine-promoted Sonogashira coupling reaction procedure did not result in any alkyne formation with chloroquinolines 12 and 14. Instead, we found that the amine undergoes nucleophilic aromatic substitution to form the corresponding 4-pyrrolidinoquinolines.23 However, the quinoline-derived alkynes 13 and 15 can be obtained in 71% and 73% yields, respectively, using aqueous 0.5 M NaOH (entries 6 and 7).
| Entry | Aryl halide | Alkyne | Product | Yield (%)a |
|---|---|---|---|---|
| a General conditions: 100 mg of 1, 2 equivalents of the terminal alkyne, 10 mol% POPd, 1.0 equivalent of TBAB, 2 equivalents of pyrrolidine, 10 mol% of CuI in 5 mL of deionized water at 135–140 °C for 5 h in a closed vessel. b Pyrrolidine was replaced by 0.5 M NaOH. c No CuI was added. | ||||
| 1 |

|

|
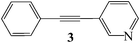
|
90 (80c) |
| 2 |

|

|

|
91(82b) |
| 3 |

|

|

|
73(64b) |
| 4 |

|

|

|
81 |
| 5 |
|

|

|
90(90b) |
| 6 |

|

|

|
71b |
| 7 |

|

|

|
73b |
| 8 |

|

|

|
65b(51c) |
| 9 |

|

|

|
90 |
| 10 |

|

|

|
70 |
| 11 |

|

|

|
81 |
Formation of 5–10% butadiynes derived from terminal alkynes 2 and 17 was observed when CuI was used as a co-catalyst. This may be attributed to oxidative Glaser coupling, which is a well known side reaction of Cu(I)-promoted coupling reactions in the presence of air. Recently, copper-free Sonogashira coupling methods using aryl bromides and iodides have been introduced.24 We observed that POPd-catalyzed Sonogashira coupling also proceeds with aryl chlorides in the absence of CuI albeit with slightly decreased yields (entries 1 and 8).
In summary, we have developed a Sonogashira coupling method that utilizes a palladium–phosphinous acid catalyst in water and avoids the use of hazardous organic co-solvents. Disubstituted alkynes have been prepared in good to high yields from aryl chlorides, bromides and iodides with POPd in the presence of TBAB. Pyrrolidine was found to afford superior results over other organic and inorganic bases but its use is limited to aryl halides that are not susceptible to nucleophilic aromatic substitution. Replacement of pyrrolidine by NaOH avoids amination of chloroquinolines and thus allows conversion of these electron-deficient aryl chlorides to alkynes in high yields. The present and previously reported studies from our laboratories show that POPd combines high catalytic activity for Stille and Sonogashira cross-coupling reactions with remarkable stability to air and water. We believe that this catalyst is also a very promising candidate for developing other C–C bond forming reactions that proceed in water and thus minimize waste production and environmental pollution.
Acknowledgements
We thank Combiphos Catalysts, Inc., New Jersey (www.combiphos.com) for POPd, POPd1 and POPd2.Notes and references
- For recent review: A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 Search PubMed; N. Miyaura, Ed, Cross-Coupling Reactions, Springer, Berlin, 2002 CrossRef CAS.
- V. P. W. Bohm and W. A. Herrmann, Chem. Eur. J., 2000, 6, 1017–1025 CrossRef CAS; A. S. Gruber, D. Zim, G. Ebeling, A. L. Monteiro and J. Dupont, Org. Lett., 2000, 2, 1287–1290 CrossRef CAS; D. Morales-Morales, R. Redon, C. Yung and C. M. Jensen, Chem. Commun., 2000, 1619–1620 RSC; V. P. W. Bohm, T. Weskamp, C. W. K. Gstottmayr and W. A. Herrmann, Angew. Chem., Int. Ed., 2000, 39, 1602–1604 CrossRef CAS; I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS; A. F. Littke, C. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020–4028 CrossRef CAS; A. Zapf, A. Ehrentraut and M. Beller, Angew. Chem., Int. Ed., 2000, 39, 4153–4155 CrossRef CAS; J. P. Stambuli, S. R. Stauffer, K. H. Shaughnessy and J. F. Hartwig, J. Am. Chem. Soc., 2001, 123, 2677–2678 CrossRef CAS; C. Dai and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 2719–2724 CrossRef CAS; A. F. Littke and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 6989–7000 CrossRef CAS; N. J. Whitcombe, K. K. Hii and S. E. Gibson, Tetrahedron, 2001, 57, 7449–7476 CrossRef CAS; J. J. Yin, M. P. Rainka, X. X. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 1162–1163 CrossRef CAS; R. B. Bedford, C. S. J. Cazin and S. L. Hazelwood, Chem. Commun., 2002, 2608–2609 RSC; R. B. Bedford, C. S. J. Cazin and S. L. Hazelwood, Chem. Commun., 2002, 2610–2611 RSC.
- C.-J. Li, Chem. Rev., 1993, 93, 2023–2035 CrossRef CAS; A. Lubineau, J. Augé and Y. Queneau, Synthesis, 1994, 741–760 CrossRef CAS; J. B. F. N. Engberts, B. L. Feringa, E. Keller and S. Otto, Recl. Trav. Chim. Pays-Bas, 1996, 115, 457–464 CAS; C. Li, T.-H. Chen, Organic Reactions in Aqueous Media, Wiley, New York, 1997 Search PubMed; B. Cornils, W. A. Herrmann and R. W. Eckl, J. Mol. Catal. A: Chem., 1997, 116, 27–33 Search PubMed; P. A. Grieco, Ed. Organic Synthesis in Water, Blackie Academic & Professional, London, 1998 CrossRef CAS; A. Lubineau and S. E. Augé, Top. Curr. Chem., 1999, 206, 1–39 CrossRef CAS; S. Kobayashi, Eur. J. Org. Chem., 1999, 15–27 Search PubMed; S. Kobayashi and K. Manabe, Pure Appl. Chem., 2000, 72, 1373–1380 Search PubMed.
- E. Paetzold and G. Oehme, J. Mol. Catal. A: Chem., 2000, 152, 69–76 CrossRef CAS; C. R. LeBlond, A. T. Andrews, Y. Sun and J. R. Sowa, Org. Lett., 2001, 3, 1555–1557 CrossRef CAS; K. H. Shaughnessy and R. S. Booth, Org. Lett., 2001, 3, 2757–2759 CrossRef CAS; C. Dupuis, K. Adiey, L. Charruault, V. Michelet, M. Savignac and J.-P. Genet, Tetrahedron Lett., 2001, 42, 6523–6526 CrossRef CAS; M. Ueda, M. Nishimura and N. Miyaura, Synlett, 2000, 856–858 CAS; L. Botella and C. Najera, Angew. Chem., Int. Ed., 2002, 41, 179–181 CrossRef CAS; L. Botella and C. Najera, J. Organomet. Chem., 2002, 663, 46–57 CrossRef CAS; Y. Uozomi and Y. Nakai, Org. Lett., 2002, 4, 2997–3000 CrossRef CAS; D. A. Alonso, C. Najera and M. A. Pacheco, J. Org. Chem., 2002, 67, 5588–5594 CrossRef CAS; R. B. Bedford, M. E. Blake, C. P. Butts and D. Holder, Chem. Commun., 2003, 466–467 RSC.
- G. Wuellner, H. Jaensch, S. Kannenberg, F. Schubert and G. Boche, Chem. Commun., 1998, 1509–1510 RSC.
- A. I. Roshchin, N. A. Bumagin and I. P. Beletskaya, Tetrahedron Lett., 1995, 36, 125–128 CrossRef CAS; R. Rai, K. B. Aubrecht and D. B. Collum, Tetrahedron Lett., 1995, 36, 3111–3114 CrossRef CAS; S.-K. Kang, T.-G. Baik and S.-Y. Song, Synlett, 1999, 3, 327–329.
- M. Murata, K. Suzuki, S. Watanabe and Y. Masuda, J. Org. Chem., 1997, 62, 8569–8571 CrossRef CAS; M. Murata, S. Watanabe and Y. Masuda, Tetrahedron Lett., 1999, 40, 9255–9257 CrossRef CAS; M. Murata, R. Shimazaki, S. Watanabe and Y. Masuda, Synthesis, 2001, 2231–2233 CrossRef CAS; S. Oi, Y. Honma and Y. Inoue, Org. Lett., 2002, 4, 667–669 CrossRef CAS; T. S. Huang and C. J. Li, Chem. Commun., 2001, 2348–2349 RSC.
- J. P. Genet, E. Blart and M. Savignac, Synlett, 1992, 715–717 CrossRef CAS.
- P. J. Genet and M. Savignac, J. Organomet. Chem., 1999, 576, 305–317 CrossRef; K. Takami, H. Yorimitsu, H. Shinokubo, S. Matsubara and K. Oshima, Org. Lett., 2001, 3, 1997–1999 CrossRef CAS; C. Najera, J. Gil-Molto, S. Karlstrom and L. R. Falvello, Org. Lett., 2003, 5, 1451–1454 CrossRef CAS.
- N. E. Leadbeater and M. Marco, J. Org. Chem., 2003, 68, 888–892 CrossRef CAS.
- L. Cassar, J. Organomet. Chem., 1975, 93, 253–257 CrossRef CAS; A. N. Tischler and T. J. Lanza, Tetrahedron Lett., 1986, 27, 1653–1656 CrossRef CAS; R. Singh and G. Just, J. Org. Chem., 1989, 54, 4453–4457 CrossRef CAS; I. Mangalagiu, T. Benneche and K. Undheim, Acta Chem. Scand., 1996, 50, 914–917 CAS; K.-W. Poon, W. Liu, P.-K. Chan, Q. Yang, T.-W. D. Chan, T. C. W. Mak and D. K. P. Ng, J. Org. Chem., 2001, 66, 1553–1559 CrossRef CAS; M. Sonoda, A. Inaba, K. Itahashi and Y. Tobe, Org. Lett., 2001, 3, 2419–2421 CrossRef CAS; N. F. Langille, L. A. Dakin and J. S. Panek, Org. Lett., 2002, 4, 2485–2488 CrossRef CAS; A. N. Thadani and V. H. Rawal, Org. Lett., 2002, 4, 4321–4323 CrossRef CAS; G. Hilt, T. J. Korn and K. I. Smolko, Synlett, 2003, 241–243 CrossRef CAS; A. Kollhofer, T. Pullmann and H. Plenio, Angew. Chem., Int. Ed., 2003, 42, 1056–1058 CrossRef CAS; S. Ma, H. Ren and Q. Wei, J. Am. Chem. Soc., 2003, 125, 4817–4830 CrossRef CAS.
- M. J. Mio, L. C. Kopel, J. B. Braun, T. L. Gadzikwa, K. L. Hull, R. G. Brisbois, C. J. Markworth and P. A. Grieco, Org. Lett., 2002, 4, 3199–3202 CrossRef CAS.
- F. Quignard, S. Larbot, S. Goutodier and A. Choplin, J. Chem. Soc., Dalton Trans., 2002, 1147–1152 RSC.
- Z. Novak, A. Szabo, J. Repasi and A. Kotschy, J. Org. Chem., 2003, 68, 3327–3329 CrossRef CAS.
- M. P. Lopez-Deber, L. Castedo and J. R. Granja, Org. Lett., 2001, 3, 2823–2826 CrossRef CAS.
- C. Amatore, E. Blart, J. P. Genet, A. Jutand, S. Lemaire-Audoire and M. Savignac, J. Org. Chem., 1995, 60, 6829–6839 CrossRef CAS.
- Y. Uozumi and Y. Kobayashi, Heterocycles, 2003, 59, 71–74 CAS.
- H. Dibowski and F. P. Schmidtchen, Angew. Chem., Int. Ed., 1998, 37, 476–478 CrossRef CAS; H. Dibowski and F. P. Schmidtchen, Tetrahedron Lett., 1998, 39, 525–528 CrossRef CAS.
- D. T. Bong and M. R. Ghadiri, Org. Lett., 2001, 3, 2509–2511 CrossRef CAS.
- G. Y. Li, Angew. Chem., Int. Ed., 2001, 40, 1513–1516 CrossRef CAS; G. Y. Li, G. Zheng and A. F. Noonan, J. Org. Chem., 2001, 66, 8677–8681 CrossRef CAS; G. Y. Li, J. Org. Chem., 2002, 67, 3643–3650 CrossRef CAS; W. Yang, Y. Wang and J. R. Corte, Org. Lett., 2003, 5, 3131–3134 CrossRef CAS.
- C. Wolf and R. Lerebours, J. Org. Chem., 2003, 68, 7077–7084 CrossRef CAS; C. Wolf and R. Lerebours, Synthesis, 2003, 2069–2073 CrossRef CAS.
- C. Wolf and R. Lerebours, J. Org. Chem., 2003, 68, 7551–7554 CrossRef CAS.
- We have previously reported POPd-catalyzed amination of chloroquinolines using pyrrolidine in DMF at 135 °C, see reference 21a.
- N. E. Leadbeater and B. J. Tominack, Tetrahedron Lett., 2003, 44, 8653–8656 CrossRef CAS; A. Soheili, J. Albanaze-Walker, J. A. Murry, P. G. Dormer and D. L. Hughes, Org. Lett., 2003, 5, 4191–4194 CrossRef CAS and references therein.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of Sonogashira coupling products. See http://www.rsc.org/suppdata/ob/b4/b407773c/ |
| This journal is © The Royal Society of Chemistry 2004 |
