Synthesis of novel selenium-containing donors as selenium analogues of diiodo(ethylenedithio)diselenadithiafulvalene (DIETS)†
Takashi
Shirahata
and
Tatsuro
Imakubo
*
Imakubo Initiative Research Unit, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: imakubo@riken.jp
First published on 17th May 2004
Abstract
Novel selenium analogues of diiodo(ethylenedithio)diselenadithiafulvalene (DIETS) have been successfully derived from 1,3-diselenole-2-thione, which could be synthesized without the use of the highly toxic reagent CSe2.
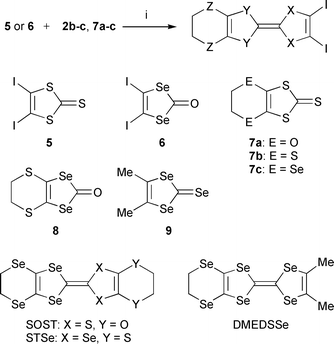
Since the first discovery of the organic superconductor (TMTSF)2PF6,1 more than one hundred organic superconductors have been discovered from cation radical salts of TTF derivatives.2 Exploration of novel TTF-based organic conductors with interesting physical properties requires designing an independent donor molecule and controlling the molecular arrangement in a crystal. For controlling the molecular arrangement, we propose a useful concept of crystal engineering3 based on an “iodine bond”,4 which is a strong and directional iodine-based intermolecular interaction. A variety of supramolecular conductors based on iodinated TTFs have been synthesized.5,6 Among them, θ-(DIETS)2[Au(CN)4] is a unique superconductor with Tc = 8.6 K (onset) under 10 kbar uniaxial strain parallel to the I⋯N iodine bonding direction.6c For designing an independent molecule, replacement of the sulfur atom in the TTF skeleton by the larger selenium atom is an effective method for the increase in electrical conductivity and development of organic superconductors. However, it is always difficult to synthesize selenium-containing donors in safety, because in most cases7 the highly hazardous CSe2 is an indispensable material for the synthesis of [1,3]diselenole-2-selone, which is a key unit for the construction of the selenium substituted skeletons. A safer synthetic method for the synthesis of the [1,3]diselenole ring has long been awaited. We have recently reported a new CSe2-free synthesis of [1,3]diselenole-2-thione 1, which may be a good alternative to [1,3]diselenole-2-selone together with its application to synthesis of iodinated [1,3]diselenole-2-thiones.8 In the course of our study to extend the synthetic application of [1,3]diselenole-2-thione, we have tried to introduce an alkylenediseleno group using conventional reagents under mild conditions. In this paper, we report a new CSe2-free synthesis of alkylenediseleno derivatives of [1,3]diselenole-2-thiones and its systematic application to the synthesis of novel selenium analogues of DIETS depicted in Fig. 1 together with their crystal structures and physical properties.
 | ||
| Fig. 1 Selenium analogues of DIETS. | ||
4,5-Ethylenediseleno-[1,3]diselenole-2-selone 2a and its methylene analogue 3a were first synthesized from [1,3]diselenole-2-selone-4,5-diselenoate, which was prepared by the electrochemical reduction of CSe2,9 however the reduction process of CSe2 is not preferable for laboratory use. Recently Otsubo et al. reported a modified synthesis of 2a and 3a using bis(selenocyanato)alkane as the electrophile from [1,3]diselenole-2-selone, which was synthesized from CSe2.10 We applied the same conditions to the synthesis of thiones 2b and 3b. Di-lithiation of 1 with 2.2 equiv. of LDA in THF at −78 °C followed by treatment with 1,2-bis(selenocyanato)ethane afforded 4,5-ethylenediselno-[1,3]thiaselenole-2-selone 4 in 20% yield and the desired thione 2b was obtained in only 3% yield. Similar transformation of the diselenole ring was observed in the iodination reaction of [1,3]diselenole-2-thione and could not be avoided even at low temperature.8,11 For the purpose of preventing unfavorable ring transformation, we changed the reaction protocol as follows: first a THF solution of a mixture of 1 and 1,2-bis(selenocyanato)ethane was prepared and then the appropriate amount of LDA was added at low temperature (Scheme 1). This inverted sequence afforded the desired thione 2b without a selenium–sulfur exchange side reaction as well as the iodination reaction. However, the yield of thione 2b remained below 5%, and much insoluble matter generated by intermolecular polymerization was produced. Considering the concentration effect of the reaction, we adopted the following dilution conditions: to a mixture of thione 1 (102 mg, 0.45 mmol) and 1,2-bis(selenocyanato)ethane (129 mg, 0.54 mmol) in dry THF (100 ml) at −78 °C was slowly added LDA (0.40 M, 2.8 ml, 1.1 mmol) during a period of 5 min to afford 4,5-ethylenediseleno-[1,3]diselenole-2-thione 2b as an ochre powder (39 mg, 21%). In this condition, the starting material 1 (43%) was recovered and generation of the insoluble matter was effectively prevented. It is easy to separate 1 and 2b by conventional silica gel column chromatography or preparative gel permeation chromatography (GPC) and the recovered 1 was recycled for the same reaction. The methylenediseleno derivative 3b was also synthesized in a similar manner, however, in contrast to the conditions for the ethylenediseleno derivative a higher concentration of the reagents (ca. 20 mmol dm−3) resulted in a good yield (34%). The intramolecular cyclization of the reaction is preferred to the intermolecular polymerizations in the case of a five-membered ring compared with a six-membered ring because of the lower strain energy of the ring. Thiones 2b and 3b were easily converted to the corresponding ketones 2c and 3c by the conventional Hg(OAc)2–CHCl3 method.
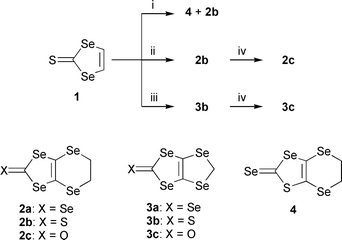 | ||
| Scheme 1 Reagents and conditions: i, LDA (2.2 eq.) then 1,2-bis(selenocyanato)ethane (1.6 eq), −78 °C, ii, 1,2-bis(selenocyanato)ethane (1.2 eq.), then LDA (2.5 eq.), −78 °C, iii, bis(selenocyanato)methane (2.0 eq.), then LDA (3.0 eq.), −95 °C, iv, Hg(OAc)2, AcOH–CHCl3 (89% for 2c, 59% for 3c). | ||
Ethylenediseleno derivatives 2b and 2c are applicable for the phosphite-mediated coupling reaction. Novel selenium analogues of DIETS and related ET and DMET analogues were synthesized by the phosphite-mediated cross-coupling reaction under the conditions listed in Table 1 (Scheme 2). It has been reported that the treatment of the [1,3]diselenole-2-thione derivatives with trialkyl phosphite produces corresponding triselenathiafulvalenes, which are sulfur–selenium scrambling products,12 however, the cross-coupling reaction of 2b and 5 provides only the expected product, DIEDSSe. It has been reported that the sulfur–selenium interchange occurs via the ring-opening reaction and it must be completely suppressed in the coupling reaction of the heterocycle-fused [1,3]diselenole-2-thiones, which cannot open the [1,3]diselenole ring. Unfortunately, no trace of coupling products was detected by the phosphite-mediated coupling reaction of methylenediseleno derivatives 3b or 3c. All molecular structures of the new compounds were characterized by NMR, MS, and elemental analyses.‡
| Donor | Materials | Solvent | Yield (%) | E 1 1/2/V | E 2 1/2/V | ΔE (= E21/2 − E11/2) |
|---|---|---|---|---|---|---|
| a vs. Cp2Fe–Cp2Fe+ couple, in PhCN with 0.1 M n-Bu4N·BF4, glassy carbon working electrode, 100 mV s−1, rt. b Data for DIETS and DIETSe were taken from references 6 and 8 respectively. c P(OEt)3, neat. | ||||||
| TTF | −0.10 | 0.32 | 0.42 | |||
| DIETSb | 5 + 8 | Toluene | 46 | 0.22 | 0.49 | 0.27 |
| DIEDSS | 5 + 2c | Toluene | 48 | 0.24 | 0.51 | 0.27 |
| DIEDO-STF | 6 + 7a | Benzene | 37 | 0.13 | 0.45 | 0.32 |
| DIET-STF | 6 + 7b | Benzene | 76 | 0.21 | 0.49 | 0.28 |
| DIEDS-STF | 6 + 7c | Benzene | 71 | 0.19 | 0.47 | 0.28 |
| DIETSeb | 6 + 8 | Toluene | 28 | 0.31 | 0.55 | 0.24 |
| DIEDSSe | 6 + 2b | Toluene | 21 | 0.29 | 0.52 | 0.23 |
| SOST | 2c + 7a | Benzene | 69 | 0.08 | 0.39 | 0.31 |
| STSe | 2b + 8 | —c | 24 | 0.21 | 0.44 | 0.23 |
| DMEDSSe | 2c + 9 | Toluene | 12 | 0.09 | 0.39 | 0.30 |
The redox potentials of new donors are summarized in Table 1 together with those of related compounds. A series of DIETS analogues exhibit two reversible redox waves and their donor abilities depend on the inner chalcogen element except for DIEDO-STF because of the strong electron-donating ability of the ethylenedioxy group. The E11/2 values of donors of diselenadithiafulvalene (DSDTF) derivative are comparable with each other and lower than those of TSeF derivative. Comparison of the ΔE values of DIEDSSe (0.23 V) and DMEDSSe (0.30 V) revealed that the on-site Coulombic repulsion is reduced by the extension of HOMO toward the iodine atom on the edge of the skeleton, and it is an advantage for the preparation of novel organic conductors with stable metallic nature.
Fig. 2 shows the crystal structure of DIEDSSe, which is the all-selenated analogue of DIETS.§ There are two crystallographically independent molecules A and B. The conformation of the TSeF skeletons for both molecules adopt a boat conformation and the folding angles are 20.4° and 20.6° for Molecule A and 14.1° and 21.9° for Molecule B, respectively. The packing motif of the molecule is well tailored by the chalcogen⋯chalcogen interactions and the strong iodine bonds. The donors face in a head-to-tail manner to avoid the steric repulsion of terminal ethylenediseleno group and two kinds of dimers that are connected by short Se⋯Se contacts (d1 = 3.652(1), d2 = 3.688(1) Å) shorter than sum of the van der Waals radii (3.80 Å).13 The dimers are also linked perpendicularly by the strong I⋯Se iodine bonds (d3 = 3.528(1), d4 = 3.512(1) Å) which is 10% less than sum of the van der Waals radii (3.88 Å). These strong interactions will be useful for crystal engineering of their cation radical salts, and research on cation radical salts of the new selenium analogues of DIETS is currently in progress.
![Crystal structure of DIEDSSe: (a) molecular structures of crystallographically independent molecules A and B; (b) crystal packing diagram viewed along the crystallographic a-axis. Thick and dotted lines indicate short Se⋯Se and I⋯Se contacts shorter than the sum of van der Waals radii, respectively [d1
= 3.652(1), d2
= 3.688(1), d3
= 3.528(1), d4
= 3.512(1)
Å].](/image/article/2004/OB/b406092j/b406092j-f2.gif) | ||
| Fig. 2 Crystal structure of DIEDSSe: (a) molecular structures of crystallographically independent molecules A and B; (b) crystal packing diagram viewed along the crystallographic a-axis. Thick and dotted lines indicate short Se⋯Se and I⋯Se contacts shorter than the sum of van der Waals radii, respectively [d1 = 3.652(1), d2 = 3.688(1), d3 = 3.528(1), d4 = 3.512(1) Å]. | ||
In summary, we developed a new synthetic route to 4,5-alkylenediseleno-[1,3]diselenole-2-thione 2b and 3b without using hazardous reagents. Ethylenediseleno derivatives 2b and 2c are useful materials for synthesizing a wide variety of DSDTFs and TSeFs. The novel selenium analogues of DIETS show smaller on-site Coulombic repulsion energy and the existence of a strong nature to construct chalcogen⋯chalcogen contacts and iodine bonds in the crystals are superior for synthesizing novel supramolecular organic conductors with interesting physical properties.
Acknowledgements
This work was partially supported by the grant-in-aid for scientific research (Nos.14740390 and 14204033) from JSPS and MEXT. We are grateful to Ms. Megumi Kibune for the assistance of the synthesis of raw materials.Notes and references
- D. Jerome, A. Mazaud, M. Ribault and K. Bechgaard, J. Phys. Lett., 1980, 41, L95–L98 Search PubMed.
- For comprehensive reviews on organic superconductors, see: T. Ishiguro, K. Yamaji and G. Saito, Organic Superconductors, Springer-Verlag, Berlin, 2nd edn., 1998 Search PubMed; J. M. Williams, R. J. Thorn, J. R. Ferraro, K. D. Carlson, U. Geiser, H. H. Wang, A. M. Kini and M.-H. Whangbo, Organic Superconductors (Including Fullerenes) Synthesis, Structure, Properties, and Theory, Prentice-Hall, New Jersey, 1992 Search PubMed.
- (a) G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam, 1989 Search PubMed; (b) G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311–2327 CrossRef CAS.
- T. Imakubo, in TTF Chemistry: Fundamentals and Applications of Tetrathiafulvalene, Halogenated TTFs, J. Yamada and T. Sugimoto, ed., Kodansha and Springer, Tokyo, 2004; Chapter 3 Search PubMed.
- (a) M. R. Bryce and G. Cooke, Synthesis, 1991, 263–265 CrossRef CAS; (b) C. Wang, A. Ellern, V. Khodorkovsky, J. Bernstein and J. Y. Becker, J. Chem. Soc., Chem. Commun., 1994, 983–984 RSC; (c) R. Gompper, J. Hock, K. Polborn, E. Dormann and H. Winter, Adv. Mater., 1995, 7, 41–43 CrossRef CAS; (d) T. Imakubo, H. Sawa and R. Kato, Synth. Met., 1995, 73, 117–122 CrossRef CAS; (e) Y. Kuwatani, E. Ogura, H. Nishikawa, I. Ikemoto and M. Iyoda, Chem. Lett., 1997, 817–818 CrossRef CAS; (f) T. Imakubo, T. Maruyama, H. Sawa and K. Kobayashi, Chem. Commun., 1998, 2021–2022 RSC; (g) K. Takimiya, Y. Kataoka, A. Morikami, Y. Aso and T. Otsubo, Synth. Met., 2001, 120, 875–876 CrossRef CAS; (h) A. S. Batsanov, M. R. Bryce, A. Chesney, J. A. K. Howard, D. E. John, A. J. Moore, C. L. Wood, H. Gershtenman, J. Y. Becker, V. Y. Khodorkovsky, A. Ellern, J. Bernstein, I. F. Perepichka, V. Rotello, M. Gray and A. O. Cuello, J. Mater. Chem., 2001, 11, 2181–2191 RSC; (i) R. Suizu and T. Imakubo, Org. Biomol. Chem., 2003, 1, 3629–3631 RSC.
- (a) T. Imakubo, H. Sawa and R. Kato, J. Chem. Soc., Chem. Commun., 1995, 1667–1668 RSC; (b) T. Imakubo, H. Sawa and R. Kato, Synth. Met., 1997, 86, 1883–1884 CrossRef CAS; (c) T. Imakubo, N. Tajima, M. Tamura, R. Kato, Y. Nishio and K. Kajita, J. Mater. Chem., 2002, 12, 159–161 RSC.
- (a) For examples of CSe2-free synthesis of TSeFs see: Y. A. Jackson, C. L. White, M. V. Lakshmikantham and M. P. Cava, Tetrahedron Lett., 1987, 28, 5635–5636 Search PubMed; (b) G. C. Papavassiliou, S. Y. Yiannopoulos, J. S. Zambounis, K. Kobayashi and K. Umemoto, Chem. Lett., 1987, 1279–1282 CAS; (c) R. Kato, H. Kobayashi and A. Kobayashi, Synth. Met., 1991, 41–43, 2093–2096 CrossRef CAS.
- T. Imakubo and T. Shirahata, Chem. Commun., 2003, 1940–1941 RSC.
- V. Y. Lee, E. M. Engler, R. R. Schumaker and S. S. P. Parkin, J. Chem. Soc., Chem. Commun., 1983, 235–236 RSC.
- A. Morikami, K. Takimiya, Y. Aso and T. Otsubo, Org. Lett., 1999, 1, 23–25 CrossRef CAS.
- H. Poleschner, R. Radeglia and J. Fuchs, J. Organomet. Chem., 1992, 427, 213–230 CrossRef CAS.
- E. M. Engler and V. V. Patel, J. Chem. Soc., Chem. Commun., 1977, 835–836 RSC.
- A. Bondi, J. Phys. Chem., 1964, 68, 411–451 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. See http://www.rsc.org/suppdata/ob/b4/b406092j/ |
‡ Selected data of new compounds: 2b: ochre powder, mp 130 °C; m/z
(EI, 70 eV): 414 (M+ for C5H4S78Se80Se3), 370 (M+
− C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); δH
(270 MHz, CDCl3) 3.47 (s, 4H); Elemental analysis: Calc. for C5H4SSe4: C, 14.58; H, 0.98. Found: C, 14.34; H, 0.82%; 3b: brown crystals, mp 185 °C (decomp.); m/z
(EI, 70 eV): 400 (M+ for C4H2S78Se80Se3), 356 (M+
− C S); δH
(270 MHz, CDCl3) 3.47 (s, 4H); Elemental analysis: Calc. for C5H4SSe4: C, 14.58; H, 0.98. Found: C, 14.34; H, 0.82%; 3b: brown crystals, mp 185 °C (decomp.); m/z
(EI, 70 eV): 400 (M+ for C4H2S78Se80Se3), 356 (M+
− C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); δH
(270 MHz, CDCl3) 4.89 (s, 2H); Elemental analysis: Calc. for C4H2SSe4: C, 12.16; H, 0.48. Found: C, 12.08; H, 0.46%; 4: purplish-brown crystals, mp 170 °C (decomp.); m/z
(EI, 70 eV): 414 (M+ for C5H4S78Se80Se3), 322 (M+
− C S); δH
(270 MHz, CDCl3) 4.89 (s, 2H); Elemental analysis: Calc. for C4H2SSe4: C, 12.16; H, 0.48. Found: C, 12.08; H, 0.46%; 4: purplish-brown crystals, mp 170 °C (decomp.); m/z
(EI, 70 eV): 414 (M+ for C5H4S78Se80Se3), 322 (M+
− C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se); δH
(270 MHz, CDCl3) 3.44 (m, 2H), 3.45 (m, 2H); Elemental analysis: Calc. for C5H4SSe4: C, 14.58; H, 0.98. Found: C, 14.59; H, 0.91%; DIEDSS: orange–brown crystals, mp 157 °C (decomp.); m/z
(EI, 70 eV): 736 (M+ for C8H4I2S278Se80Se3); δH
(270 MHz, CDCl3) 3.37 (s, 4H); Elemental analysis: Calc. for C8H4I2S2Se4: C, 13.09; H, 0.55. Found: C, 13.14; H, 0.53%; DIEDO-STF: reddish-brown crystals, mp 178 °C (decomp.); m/z
(EI, 70 eV): 610 (M+ for C8H4I2O2S280Se2); δH
(270 MHz, CDCl3) 4.26 (s, 4H); Elemental analysis: Calc. for C8H4I2O2S2Se2: C, 15.80; H, 0. 66. Found: C, 15.84; H, 0.60%; DIET-STF: purplish-brown crystals, mp 175 °C (decomp.); m/z
(EI, 70 eV): 642 (M+ for C8H4I2S480Se2); δH
(270 MHz, CDCl3) 3.30 (s, 4H); Elemental analysis: Calc. for C8H4I2S4Se2: C, 15.01; H, 0.63. Found: C, 14.96; H, 0.53%; DIEDS-STF: orange–brown crystals, mp 154 °C (decomp.); m/z
(EI, 70 eV): 736 (M+ for C8H4I2S278Se80Se3); δH
(270 MHz, CDCl3) 3.35 (s, 4H); Elemental analysis: Calc. for C8H4I2S2Se4: C, 13.09; H, 0.55. Found: C, 13.16; H, 0.50%; DIEDSSe: orange plates, mp 167 °C (decomp.); m/z
(EI, 70 eV): 830 (M+ for C8H4I278Se280Se4); δH
(270 MHz, CDCl3) 3.38 (s, 4H); Elemental analysis: Calc. for C8H4I2Se6: C, 11.61; H, 0.49. Found: C, 11.71; H, 0.44%; SOST: orange crystals, mp 209 °C (decomp.); m/z
(EI, 70 eV): 542 (M+ for C10H8O2S278Se80Se3); δH
(270 MHz, CDCl3) 3.36 (s, 4H, SCH2CH2S), 4.26 (s, 4H, OCH2CH2O); Elemental analysis: Calc. for C10H8O2S2Se4: C, 22.24; H, 1.49. Found: C, 22.26; H, 1.44%; STSe: reddish-purple crystals, mp 280 °C (decomp.); m/z
(EI, 70 eV): 668 (M+ for C10H8S278Se280Se4); δH
(270 MHz, CDCl3) 3.29 (s, 4H), 3.36 (s, 4H); Elemental analysis: Calc. for C10H8S2Se6: C, 18.03; H, 1.21. Found: C, 18.08; H, 1.19%; DMEDSSe: orange crystals, mp 227 °C (decomp.); m/z
(EI, 70 eV): 606 (M+ for C10H1078Se280Se4); δH
(270 MHz, CDCl3) 2.00 (s, 6H), 3.36 (s, 4H); Elemental analysis: Calc. for C10H10Se6: C, 19.89; H, 1.67. Found: C, 19.89; H, 1.55%. Se); δH
(270 MHz, CDCl3) 3.44 (m, 2H), 3.45 (m, 2H); Elemental analysis: Calc. for C5H4SSe4: C, 14.58; H, 0.98. Found: C, 14.59; H, 0.91%; DIEDSS: orange–brown crystals, mp 157 °C (decomp.); m/z
(EI, 70 eV): 736 (M+ for C8H4I2S278Se80Se3); δH
(270 MHz, CDCl3) 3.37 (s, 4H); Elemental analysis: Calc. for C8H4I2S2Se4: C, 13.09; H, 0.55. Found: C, 13.14; H, 0.53%; DIEDO-STF: reddish-brown crystals, mp 178 °C (decomp.); m/z
(EI, 70 eV): 610 (M+ for C8H4I2O2S280Se2); δH
(270 MHz, CDCl3) 4.26 (s, 4H); Elemental analysis: Calc. for C8H4I2O2S2Se2: C, 15.80; H, 0. 66. Found: C, 15.84; H, 0.60%; DIET-STF: purplish-brown crystals, mp 175 °C (decomp.); m/z
(EI, 70 eV): 642 (M+ for C8H4I2S480Se2); δH
(270 MHz, CDCl3) 3.30 (s, 4H); Elemental analysis: Calc. for C8H4I2S4Se2: C, 15.01; H, 0.63. Found: C, 14.96; H, 0.53%; DIEDS-STF: orange–brown crystals, mp 154 °C (decomp.); m/z
(EI, 70 eV): 736 (M+ for C8H4I2S278Se80Se3); δH
(270 MHz, CDCl3) 3.35 (s, 4H); Elemental analysis: Calc. for C8H4I2S2Se4: C, 13.09; H, 0.55. Found: C, 13.16; H, 0.50%; DIEDSSe: orange plates, mp 167 °C (decomp.); m/z
(EI, 70 eV): 830 (M+ for C8H4I278Se280Se4); δH
(270 MHz, CDCl3) 3.38 (s, 4H); Elemental analysis: Calc. for C8H4I2Se6: C, 11.61; H, 0.49. Found: C, 11.71; H, 0.44%; SOST: orange crystals, mp 209 °C (decomp.); m/z
(EI, 70 eV): 542 (M+ for C10H8O2S278Se80Se3); δH
(270 MHz, CDCl3) 3.36 (s, 4H, SCH2CH2S), 4.26 (s, 4H, OCH2CH2O); Elemental analysis: Calc. for C10H8O2S2Se4: C, 22.24; H, 1.49. Found: C, 22.26; H, 1.44%; STSe: reddish-purple crystals, mp 280 °C (decomp.); m/z
(EI, 70 eV): 668 (M+ for C10H8S278Se280Se4); δH
(270 MHz, CDCl3) 3.29 (s, 4H), 3.36 (s, 4H); Elemental analysis: Calc. for C10H8S2Se6: C, 18.03; H, 1.21. Found: C, 18.08; H, 1.19%; DMEDSSe: orange crystals, mp 227 °C (decomp.); m/z
(EI, 70 eV): 606 (M+ for C10H1078Se280Se4); δH
(270 MHz, CDCl3) 2.00 (s, 6H), 3.36 (s, 4H); Elemental analysis: Calc. for C10H10Se6: C, 19.89; H, 1.67. Found: C, 19.89; H, 1.55%. |
| § Crystal data forDIEDSSe: C8H4I2Se6, M = 827.67, orange plate (0.50 × 0.20 × 0.08 mm), monoclinic, P21/c (#14), a = 6.9162(12), b = 21.895(4), c = 20.761(3) Å, β = 91.611(4)°, V = 3142.6(9) Å3, μ = 17.874 mm−1, Z = 8, 23147 reflections measured, 7789 unique (Rint = 0.0554). Final R indices [I > 2σ(I)]: R1 = 0.0537, wR2 = 0.1321. R indices (all data): R1 = 0.0706, wR2 = 0.1422, GOF = 1.008. CCDC reference number 236916. See http://www.rsc.org/suppdata/ob/b4/b406092j/ for crystallographic data in .cif or other electronic format. |
| This journal is © The Royal Society of Chemistry 2004 |