Hydrodynamic microfabrication via “on the fly” photopolymerization of microscale fibers and tubes
Wonje
Jeong
a,
Jeongyun
Kim
a,
Sunjeong
Kim
b,
Sanghoon
Lee
*a,
Glennys
Mensing
c and
David. J.
Beebe
*c
aDepartment of Biomedical Engineering, Dankook University, San 29, Anseodong Cheonan Chungnam, South Korea. E-mail: dbiomed@dankook.ac.kr
bDepartment of Biomedical Engineering, Hanyang University, 17, Hangdang Seongdonggu, , Seoul, S. Korea. E-mail: sjk@hanyan.ac.kr
cDepartment of Biomedical Engineering, University of Wisconsin, Madison, WI 53706, USA. E-mail: djbeebe@wisc.edu
First published on 11th November 2004
Abstract
A microfluidic apparatus capable of creating continuous microscale cylindrical polymeric structures has been developed. This system is able to produce microstructures (e.g. fibers, tubes) by employing 3D multiple stream laminar flow and “on the fly” in-situ photopolymerization. The details of the fabrication process and the characterization of the produced microfibers are described. The apparatus is constructed by merging pulled glass pipettes with PDMS molding technology and used to manufacture the fibers and tubes. By controlling the sample and sheath volume flow rates, the dimensions of the microstructures produced can be altered without re-tooling. The fiber properties including elasticity, stimuli responsiveness, and biosensing are characterized. Responsive woven fabric and biosensing fibers are demonstrated. The fabrication process is simple, cost effective and flexible in materials, geometries, and scales.
Introduction
Strings and tubes are perhaps the most common curved objects in both the natural and man-made world, enabling a plethora of functions across diverse fields. At the microscale, they have many potential uses in chemical, biological, and industrial applications.1–3 Recent advances in man-made microscale systems have largely relied on planar two- and three-dimensional (3D) geometries inherent to integrated circuit derived processes. Recent soft material-based micro systems have provided alternatives in functionality via elastomeric4 and stimuli responsive materials,5 but their origin is still rooted in pseudo 3D constructs. Three-dimensional geometries can be achieved using gray scale lithography,6 surface tension effects,7 or multiphoton illumination.8 For the creation of microscale strings and tubes, extrusion/casting,9 layering,10 or fugitive3 processes have been used. While these approaches have merit, all have limitations associated with solid–solid interaction and extraction effects for the production of micron-scale tubes or fibers in continuous lengths. Previously, we have demonstrated the use of surface tension to create curved micro structures.11 While the use of surface tension allows for parallel fabrication (e.g. arrays) it precludes continuous sequential production. In nature, spiders elegantly produce micron-scale fibers by generating a liquid that solidifies when exposed to air. Here we present an analogous process replacing spontaneous solidification via exposure to air with light initiated solidification.In this paper, a microfluidic apparatus has been developed that creates a continuous process for the production of microscale cylindrical polymeric structures (e.g., fibers, tubes) by employing 3D multiple stream laminar flow12,13 and “on the fly” in-situ photopolymerization.14 With this apparatus, microstructures having diverse shapes such as fibers or tubes are generated by changing the channel configuration, and their dimensions can be altered by controlling the relative flow rates without re-tooling. The characterization of the properties, performance, and potential application of the microfibers are also addressed. First, the stress to strain relation of the microfiber is measured. Second, the use of stimuli responsive fibers is described. The polymerized fibers respond to pH variation with a volume transition from the collapsed to the expanded state. Stimuli responsive materials have many potential applications as efficient and robust actuating elements via direct chemical to mechanical energy transduction. The dynamic volume change of the fiber to a step pH variation is measured by using a microfluidic chamber. Third, the ability to create biosensing fibers is demonstrated. One important attribute of the method is the ability to immobilize biocatalysts into the fiber to create biosensors. We immobilized glucose reactive enzymes into the microfiber, and evaluated the sensitivity to glucose, demonstrating the mass production of a fiber that can be used as a biosensor component whose shape and size can be easily altered. Finally, other applications made possible via the local control of the fiber's physical and chemical properties are described for combinatorial sensing.
Principles and theory
The microfiber fabrication apparatus is fabricated by incorporating pulled glass micropipettes into a preformed hole in a PDMS substrate containing a microchannel network and its schematic is illustrated in Fig. 1a. Into the center hole, the pulled micropipette (for inlet channel) is incorporated for the delivery of sample fluid, while the normal (non-pulled) micropipette is positioned at the opposite side of the hole as an outlet channel. | ||
| Fig. 1 The basic apparatus for fabricating microfibers is shown in (a). By adding an additional stage, the apparatus is capable of producing microtubes (b) | ||
The photopolymerizable sample fluid (4-hydroxybutyl acrylate (4-HBA) mixed with dye and non-polymerizable sheath fluid (50 vol% polyvinyl alcohol (PVA) + 50 vol% DI water (DI = deionized)) are introduced into the two input ports of the system and combined at the ‘X’ position of the apparatus. Facilitated by phenomena that dominate at the microscale (e.g. laminar flow, diffusion), a 3-D coaxial sheath flow stream around the sample flow is formed at the merging position of both flows. Next, the outlet tube is exposed to ultraviolet (UV) (365 nm) radiation to create “on the fly” photopolymerization (i.e. continuous radiation of a moving liquid) of the sample stream as it flows towards the outlet of the channel. A micrograph of polymerized sample and sheath flows inside the outlet pipette are shown in Fig. 1a. The polymerized fiber moves along the direction of flow without touching the inner surface, emerging from the device as a polymerized fiber.
In a similar way, microtubes can be fabricated by using the apparatus shown in Fig. 1b. Into the inlet ‘A’ and ‘B’, the core fluid (25 vol% PVA + 75 vol% DI water) and sample fluid (4-HBA) are introduced respectively, and a core surrounded by sample flow is produced at the position ‘C’, as shown in Fig. 1b. The core/sample flow then enters into the second stage, where a sheath fluid (50 vol% PVA + 50 vol% DI water) is introduced producing a core/sample/sheath flow construct. via continuous UV exposure, the polymerized tubes are continuously formed.
The radius of the sample stream within a sheath flow can be described as a function of the volume flow rates of the sample and sheath streams.15,16 The coaxial flow of appropriate parameters (e.g., viscosity, flow rate, channel dimension) produces a laminar flow profile. The linear velocity, V(r), for laminar flow in a circular outlet tube is given as a function of distance to the stream center, r, where R is the tube radius.
 | (1) |
The total volume flow rate, Qtotal, is given by the sum of the sample volume flow rate, Qsample, and the sheath volume flow rate, Qsheath. Since the sample stream is circular and centered in the tube, the sample stream volume flow rate must equal the integral of eqn. (1) over the area of the sample stream. Performing the integration and solving for the sample radius, Rs yields the following equation for the radius of sample flow as a function of the sample stream and total volume flow rates:
 | (2) |
Experiments
The apparatus used to manufacture the fibers and tubes is constructed using established methods of pipette pulling and PDMS molding. Fig. 2 demonstrates the process of fabricating the apparatus used to manufacture microfibers and microtubes. A glass micropipette (Aluminosilicate glass/1714, Corning), the length of which is 4 cm, and inner and outer diameters are 0.5 mm and 1 mm, respectively, is used to mold the center hole (Fig. 2). By using double sided tape, both ends of the pipette are attached onto the acrylic anchor fixed in the Petri dish. A mixture of PDMS pre-polymer and curing agent (Sylgard 184 silicone elastomer kit, Dow Corning, Midland, MI) is prepared in a 10 : 1 ratio and poured over the pipette and anchors, and cured 2 h at 80 °C on a hot plate. The Petri dish is removed and the embedded pipette is carefully extracted. The cured PDMS substrate with center hole is cut in a suitable size. | ||
| Fig. 2 The fabrication process for making the PDMS substrate. A glass pipette is used to mold the main flow channel. | ||
A micropipette puller (P-97, Sutter Instrument Co.) is employed to make the pulled micropipette, and its tip is cut in the range of 20–40 µm using a microforge (MF-900, Narishige). The prepared PDMS substrate and pulled micropipette are exposed to an oxygen plasma, and combined by inserting the pulled pipette into the center hole using methanol as a lubricant, and bonded at the appropriate position by curing for 4 h at 80 °C. The hole for the sheath flow is cored out using a 12 gauge needle. At the opposite entrance of the center hole, a normal (non-pulled) micropipette is inserted and bonded in the same way providing an outlet for the polymerized fiber. The device for microtube production is made in a similar way. Another intermediate pulled pipette (length: 3 mm, tip diameter: 40 µm) is placed between the inlet and outlet pipette, and a hole for the sample and sheath flow delivery is cored out using a needle.
The fabricated apparatus is completely shielded by aluminum foil to prevent UV light exposure onto the channel excluding the outlet pipette. A 365 nm UV source (Novacure, EXFO) was used to expose the moving fluid with an intensity of 10.47 mW cm−2 at the surface of the outlet pipette.
Results and discussion
A. Experimental configuration
The photopolymerizable sample fluid is a mixture of 4-hydroxybutyl acrylate (85 wt.%, Sigma-Aldrich), acrylic acid (11wt.%, Sigma-Aldrich), ethyleneglycol dimethacrylate (1 wt.%, Sigma-Aldrich), and 2,2′-dimethoxy-2-phenyl-acetonephenone (3 wt.%, Sigma-Aldrich). A mixture of PVA and DI water was used for the core and sheath flows to match the viscosity of the sample flow. Ethyl vinyl acetate (EVA) tubes are utilized to form the fluidic networks, and the core, sample and sheath flows are regulated separately via infusion pumps. To enhance visualization of the sample flow, a dye (Rhodamin B) is mixed with the 4-BHA. The flow inside the outlet channel is monitored using optical microscopy, and scanning electron microscopy (S-4300, Hitachi) is employed to inspect the produced fibers and tubes. To introduce the fluids into each inlet, the syringe pumps are utilized.B. Fabricated fibers and tubes
The continuously emerging microfibers are collected into the small plastic bowl and the PVA on the fiber's surface is washed using DI water. Fig. 3a shows a continuous length of fiber produced using the apparatus. The diameter of the fiber is determined by the regulation of the sample and sheath flow rates. Fig. 3b demonstrates the relationship between the diameter and flow rates obtained from the experiment and compared to the analytical model (eqns. (1) and (2)). By changing the sample flows (1.2 and 2.4 μl min−1) and the sheath flows (50, 100, 200, and 400 μl min−1), polymerized fibers with different sizes are produced, and their diameters are measured under the optical microscope. The small differences between experimental and simulation are likely due to measurement error, fluctuations in flow rates, and shrinkage during polymerization. The microtube is also continuously fabricated and cut using a razor blade to inspect the cross section. An SEM image of the cross section is shown in Fig. 4a. The volume flow rates for core, sample and sheath streams are 2, 4 and 300 µl min−1, respectively, and the hole is clearly seen at the center of tube. The outer and inner diameters of the tube shown in Fig. 4b are 35 and 7 μm, respectively.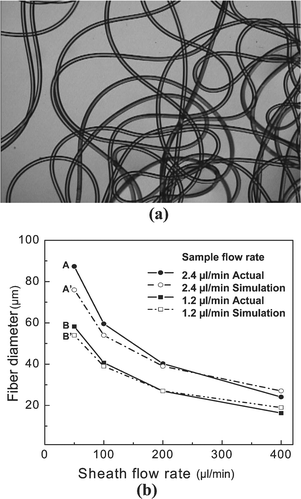 | ||
| Fig. 3 (a) A photo of continuously produced microfiber. (b) The fiber diameter is a function of the relative sample and sheath flow rates. | ||
 | ||
| Fig. 4 SEM photos of microtubes showing a whole tube (a) and a close up of the lumen (b). | ||
C. Characterization of the fiber
To measure the microfiber's elasticity, the fiber is cut to a length of 1.0 cm. One end of the fiber is fixed onto the acryl plate and an S-shaped metal hook (weight: 100 μg) is hung on the other end of the fiber. The 100 μg metal wires are prepared and bended in an S-shape, and utilized as a weighing mass by sequentially hanging additional wires on the hook. The changes in length of the microfiber are measured using a stereoscope, and the resulting stress–strain curves are shown in Fig. 5. The fiber can be stretched more than 2 times without breakage. The produced fibers are networked structures of polymer chains crosslinked to each other, and capable of undergoing large reversible deformations in response to pH changes. To measure the dynamic response due to a pH change, the fibers (diameter: 54 μm, length: 1.2 mm) are incorporated into a PDMS based microchamber (width: 0.6 mm, length: 3 mm, height: 200 μm) and a continuous flow of the buffer solution is maintained in order to minimize the effects of boundary layer resistances and to refresh the buffer solution. The fiber sizes (length and diameter) are monitored simultaneously per minute by using a stereoscope until an equilibrium volume change is reached. Fig. 6 illustrates the kinetics of swelling and shrinking of the fiber in response to step changes from pH 2.0 to 13.0. The swelling and shrinking of the fiber in response to pH changes are reversible and repeatable. | ||
| Fig. 5 The stress–strain curve of a microfiber. | ||
 | ||
| Fig. 6 Swelling and shrinking motion of a microfiber in response to a step change in pH. | ||
D. Local control of the fiber's property
A unique aspect of this process is the use of laminar flow and diffusion to enable the local control of the physical and chemical properties of the resultant fibers and tubes. This concept is demonstrated by using two different fluids (one containing a dye and one without the dye) to form the sample stream with different materials (Fig. 7a). Two 4-HBA samples (one is colored and the other is uncolored) are introduced into the inlets ‘A’ and ‘B’, and ‘sample A/sample B’ flows are produced at ‘C’ position in a cylindrical shape. The ‘sample A/sample B’ is polymerized by UV exposure, and the resultant fiber contains a gradient of this dye, as shown in Fig. 7b.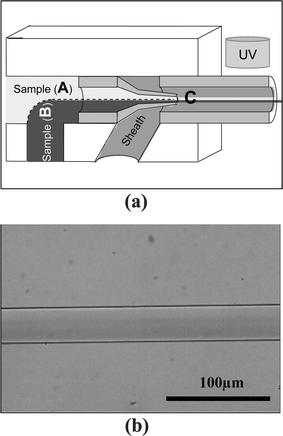 | ||
| Fig. 7 To control the local fiber properties, laminar flow (a) is used to create two adjacent materials. As a demonstration, a dye is used and the result is a fiber that contains a gradient of dye across the fiber (b). | ||
E. Enzyme immobilization
The ability to incorporate biosensing mechanisms into the fabrication process involves introduction of enzymes into the flow stream. Glucose sensing microfibers have been manufactured by entrapping two enzymes (glucose oxidase (GOX) and horseradish peroxide (HRP)) within the fiber. The 4-HBA solution is mixed with the enzymes and introduced into the apparatus to produce a biosensing microfiber. Two types of microfibers (with and without enzymes) are placed onto a slide glass. Aqueous solutions containing 20 mM glucose, 50 mM Tris-HCI buffer (pH 7.4), and 10 μM amplex red (Molecular Probes Inc., Eugene, OR) are dropped onto the fibers. The fluorescence micrograph of the microfiber is captured by the fluorescent microscope and illustrated in Fig. 8. The fluorescence was produced by exciting GOX-HRP embedded within the microfiber. | ||
| Fig. 8 Images of biosensing microfiber. (a) Optical micrograph of fiber with and without enzymes. (b) Fluorescent image showing response in fiber with enzymes and no response in fiber without enzymes. | ||
Discussion
The hydrodynamic fabrication method via ‘on-the-fly’ photopolymerization has a number of advantages over other methods for producing microstructures. The first advantage is that cylindrical microstructures can be produced continuously in a simple and cost effective way. The flexibility of the method across different materials, geometries, and scales is also one of the key advantages over existing methods that require some form of re-tooling to realize different outcomes. The second is that the fabrication process is performed under ambient conditions, and the fluid is not exposed to high voltage or high stress. In addition, the UV exposure time is very brief (e.g., 1 s). This means that biological materials such as enzymes, proteins, DNA or cells can be easily immobilized into the microstructures for use in sensing and tissue engineering applications. By cutting the functionalized fiber into sections, one could rapidly produce many biosensing components. Similarly, one could entrap proteins or cell adhesion peptides for drug delivery and tissue engineering applications, respectivelyThe stimuli-responsive ability of the isolated microscale fiber (and microtube) has many potential applications. The dynamic response time of the fiber to the pH change is fast (more than 85% of swelling and shrinking of the microfiber in length and diameter is accomplished within 1 min) due to diffusion at the microscale. This fiber having fast response time can be used as actuator elements in a microscale valve or pump without the integrated electrical components and external power supply. As depicted in Fig. 5, the stress and strain curve demonstrates that the fabricated microfiber is very elastic and shows mechanical properties with other similar polymers.17 We expect that the mechanical property (e.g., increase of tensile strength or stiffness) can be modified by altering the existing concentrations of acrylic acid and crosslinker or adding other materials like styrene.18,19 The produced fiber could be utilized to create highly stretchable fabrics with various mechanical characteristics. 4-HBA is used because of its fast photopolymerization time. Based on the flow velocity and length of the outlet pipette, the polymerization time is below 1 s. In an initial work, the differences in viscosity between the sample and water (initially used as the sheath/core fluid) caused unsteady interfaces that obstructed the formation of a coaxial sheath flow around the sample stream. To overcome the viscosity difference between sample and sheath flow, the mixture of water and PVA is introduced as a sheath flow. The PVA does not respond to 4-HBA chemically or under UV light, and is easily mixed and washed out by water or methanol.
The continuous nature of the process will allow one to scale a simple responsive swatch (Fig. 9) into cloth. In addition, the process allows for the incorporation of local functionality via parametric control combined with upstream microfluidic processing. For example, a “T” channel junction upstream at the inlet enables the creation of local packets within the sample fluid streams providing chemical and physical control of the fibers/tubes along their length while diffusion/gradients (Fig. 7b) allow control along their width.
 | ||
| Fig. 9 An example of a responsive swatch of fabric woven out of microfibers in a (a) unswollen state, (b) swollen state. | ||
Several research groups have realized sheath flow on a chip, but most of them are limited to a semi-sheath flow, consisting of a layered-flow configuration. In most cases, the sample liquid is confined in one dimension between the sheath liquid and the wall of the physical channel,20 or between two layers of sheath liquid,21 so these are not sheath flows in the classical sense. Other groups have realized coaxial sample and sheath flow in a chip, but complicated fabrication and an additional flow control scheme are required.22,23 The microfluidic chip fabrication process presented here is simple and cost effective, and the flow control scheme is straightforward compared with other methods and the weaving of responsive cloth.
Conclusion
A new method to produce continuous microscale fibers and tubes by the hydrodynamic fabrication method via ‘on-the-fly’ photopolymerization is developed, and the fiber's properties are evaluated. This spider-like fabrication process is conceptually simple, cost effective and provides flexibility in materials, geometries, and scales. While typical extrusion processes use high pressure and high temperature to extrude fibers from an orifice, our process uses multiple fluid streams at low pressure and room temperature avoiding many of the limitations inherent to extrusion while adding the local control afforded via microfluidics. Enzymes can be easily immobilized into the fluid stream, to create biosensing fibers. Responsive structures can be used as individual microscale sensing or actuating components in microdevices. By controlling the flow parameters (steady and time varying hydrodynamic focusing), polymerization parameters (time, distance, intensity), and material parameters (stimuli responsive, viscosity), a wide variety of physical and chemical properties (chemical composition, geometry, scale) can be achieved using a single microfluidic manufacturing system.References
- S-H. Su, R. Y. N. Chao, C. L. Landau, K. D. Nelson, R. B. Timmons, R. S. Meidell and R. C. Eberhart, Ann. Biomed. Eng., 2003, 31, 667–677 CrossRef.
- S. Kidoaki, I. K. Kwon and T. Matsuda, Biomaterials, 2004, 26(1), 37–46 CrossRef.
- W. P. Hoffman, H. T. Phan and P. G. Wapner, Mater. Res. Innovat., 1998, 2, 87–96 Search PubMed.
- M. A. Unger, H. Chou, T. Thorsen, A. Scherer and S. R. Quake, Science (Washington, D. C.), 2000, 288, 113–116 CrossRef CAS.
- I. Y. Galaev and B. Mattiasson, Trends Biotechnol., 1999, 17, 335–340 CrossRef CAS.
- C. Chen, D. Hirdes and A. Folch, PNAS, 2003, 100, 1499–1504 Search PubMed.
- J. M. TimesBauer, T. A. Saif and D. J. Beebe, “Surface tension driven formation of microstructures”, J. Microelectromech. Syst., 2004, 13, No. 4 in press.
- S. Kawata, H. Sun, T. Tanaka and K. Takada, Nature (London), 2001, 412, 697–698 CrossRef CAS.
- P. D. Dalton, L. Flynn and S. C. Shoichet, Biomaterials, 2002, 22, 3843–3851 CrossRef.
- S. Y. Chou, P. R. Krauss and P. J. Renstrom, Science (Washington, D. C.), 1996, 272, 85–87 CrossRef CAS.
- J. M. Bauer, T. A. Saif and D. J. Beebe, J. Microelectromech. Syst., 2004, 13(4), 553–558 CrossRef.
- Y. Kikutani, H. Hisamoto, M. Tokeshi and T. Kitamori, Lab Chip, 2004, 4, 328 RSC.
- P. J. Kenis, R. F. Ismagilov and G. M. Whitesides, Science (Washington, D. C.), 1999, 285, 83–85 CrossRef CAS . ★ This paper was the first to highlight the idea of flow separation in the micro channel, and micro fabrication inside the microfluidic channel.
- W. J. Jeong, G. Mensing, S. H. Lee and D. J. Beebe, Proc. of microTAS2004, 2004, 2, 533–535 Search PubMed.
- F. Zarrin and N. J. Dovichi, Anal. Chem., 1985, 57, 2690–2692 CrossRef CAS.
- V. Kachel and E. Menke, Flow Cytometry and Sorting, Wiley Press, New York, 2nd edn., 1991 Search PubMed.
- R. H. Liu, Q. Yu and D. J. Beebe, J. Microelectromech. Syst., 2002, 11, 45–53 CrossRef CAS.
- B. D. Johnson, W. C. Crone and D. J. Beebe, Mater. Sci. Eng., C, 2003, 24, 575–581 Search PubMed.
- J. Wootthikanokkhan and B. Tongrubbai, J. Appl. Polym. Sci., 2003, 88, 921–927 CrossRef CAS.
- M. L. Cerrada, J. L. de la Fuente, M. Fernandez-Garcia and E. L. Madruga, Polymer, 2001, 42, 4647–4655 CrossRef CAS.
- O. Hofmann, P. Niedennann and A. Manz, Lab Chip, 2001, 1, 108–114 RSC.
- G. Lee, B. Hwei and G. Huang, Proceedings of Transducers ′01, Munich, Germany, 2001, pp. 1158–1161 Search PubMed.
- J. H. Nieuwenhuis, J. Bastemeijer, P. M. Sarro and M. J. Vellekoop, Lab Chip, 2003, 3, 56–61 RSC.
| This journal is © The Royal Society of Chemistry 2004 |
