Sequence-selective DNA detection using multiple laminar streams: A novel microfluidic analysis method
Kenichi
Yamashita
,
Yoshiko
Yamaguchi
,
Masaya
Miyazaki
,
Hiroyuki
Nakamura
,
Hazime
Shimizu
and
Hideaki
Maeda
*
Micro-space Chemistry Laboratory, National Institute of Advanced Science and Technology, 807-1 Shuku-machi, Tosu, Saga 841-0052, Japan. E-mail: maeda-h@aist.go.jp; Fax: (+81) 942 81 3676; Tel: (+81) 942 81 3657
First published on 2nd December 2003
Abstract
On-site detection methods for DNA have been demanded in the pathophysiology field. Such analysis requires a simple and accurate method, rather than high-throughput. This report describes a novel microfluidic analysis method and its application for simple sequence-selective DNA detection. The method uses a microchannel device with a serpentine structure. Sequence-specific binding of probe DNA can be detected at one side of the microchannel. This method is capable of sequence-specific detection of DNA with high accuracy. Single base mutations can also be analyzed. Combination of laminar stream and laminar secondary flow in the microchannel enable specific detection of probe-bound DNA.
Introduction
Pathophysiological analysis requires a simple and accurate method, rather than high-throughput.1 Current DNA analysis methods utilize an enzyme reaction2,3 or DNA probe.4,5 The latter technique is especially suitable for rapid DNA detection.1 Several methods based on this technique have been developed using a groove binder6 or intercalator1,7–9 as the hybridization indicator. However, these methods have not been applied generally to pathophysiology because of technical difficulty, limited applications, low accuracy, and high cost.10,11We have addressed microfluidic systems to develop a simple and accurate method for quick DNA analysis. Microfluidic systems offer superior controllability of fluidics.12–14 The fluid forms a laminar stream at the straight part, whereas laminar secondary flow occurs at the turning point of a microchannel. This “laminar secondary flow” is the perpendicular flow to the principal laminar stream along the channel, and controlled easily by the channel structure and flow speed. Thus, we can create any kind of fluidic system by changing the channel structure.
Experimental
Microfluidic system
The acrylic microfluidic system chip (3 cm × 7 cm) used in this study was prepared by mechanical fabrication methods as reported in the previous paper.15 The microchannel on this chip has a cross-sectional plane whose size is 300 µm width and 200 µm depth, 2.0 mm diameter curve and 24 cm length (for Fig. 3A), or 1.5 mm diameter curve and 49 cm length (for Fig. 4A).Chemicals
All probe and target oligonucleotides were obtained from Qiagen K. K. (Japan). The probe DNA was labeled by FITC at the 5′-end. Target DNAs 1–4 have a complementary sequence to probe DNA, 5 and 6 have a one-base mismatch mutation sequence, and 7 was prepared as a negative control. Sequences corresponding to probe DNA and target DNAs 1–6 were designed using part of a unique sequence of exon 4, on which the P72/R72 SNP resides, of cancer repression gene p53.16 Concentrations of these oligonucleotides were estimated from the molar absorptivities at 260 nm:17–19 171![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 cm−1 M−1 for probe DNA, 98
800 cm−1 M−1 for probe DNA, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 cm−1 M−1 for target 1, 144
300 cm−1 M−1 for target 1, 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 M−1 for target 2, 186
600 cm−1 M−1 for target 2, 186![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1 for target 3, 648
000 cm−1 M−1 for target 3, 648![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 M−1 for target 4, 101
600 cm−1 M−1 for target 4, 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 cm−1 M−1 for target 5, 189
800 cm−1 M−1 for target 5, 189![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1 M−1 for target 6 and 243
500 cm−1 M−1 for target 6 and 243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 cm−1 M−1 for target 7.
400 cm−1 M−1 for target 7.
Apparatus
A syringe pump (KDS230; Kd Scientific, MA) controlled all injections of solutions into the microchannel. Fluorescence intensity in sequence-selective DNA sensing was measured using fluorescence microscopy with a fluorometer (C7473; Hamamatsu Photonics K.K., Japan), Ar-gas laser (Stabilite 2017, Spectra-physics Inc., CA) and a longpass filter (OG515; Edmund Industrial Optics Co. Ltd., NJ).Sequence-selective DNA detection
Probe and target DNA solutions were charged into the microchannel by syringe pumping simultaneously. Then fluorescence intensity of target solution side and probe solution sides were measured near the outlet of the straight part of the microchannel using the above described fluorescence microscopy. Detection was evaluated with a ΔF value.Results and discussion
Fig. 1 outlines the analysis system. Two solutions, probe and target DNA solutions, were charged into the microchannel simply by syringe pumping. In a straight microchannel, mixing the two solutions simply depends on diffusion (ca. 1 µm s−1). Therefore, double strands of target and probe DNA were formed around the interface area. The molecular weight increases by double strand formation; thereby the molecule localizes at the interface. At the curving part of the microchannel, internal force of the fluid produces laminar secondary flow within the channel. Such laminar secondary flow at the turn disrupts the interface and moves double-stranded DNA molecules to the outer side. When solutions were charged with double-stranded DNA, as shown in Fig. 1, we only have to compare the ΔF value, which is the background (B0/A0) subtracted ratio of fluorescence intensity at the probe DNA solution side (A) and target DNA solution side (B) of the microchannel. (A0 and B0 are the fluorescence intensities in the case of buffer solution instead of target DNA solution). | ||
| Fig. 1 Schematic principle and procedure of the sequence-specific DNA detection in the microfluidic system. | ||
The microchannel design is very important for this analysis method. The microchannel design in this study has been optimized by advance examination.
Typical results were obtained using DNA molecules as shown in Fig. 2. First, we examined which position of the channel is suitable for detection (Fig. 3A, position a–e), using complementary sequence 3 and negative control 7. Fig. 3B shows that the complementary target gave higher intensity ratios than those of the negative control at positions c and e. On the other hand, such a difference was not observed at positions b and d. These results mean that specific detection of double-stranded DNA was enabled at the points after curves of even numbers. This can be explained by simulation of microfluidics. Laminar secondary flow disrupted the interface at the first curve, but the structural interface was restored by laminar secondary flow with opposite direction at the next curve. Thus, the difference could be observed at positions c and e, whereas no difference was obtained at positions b and d.
 | ||
| Fig. 2 Structures of probe and target DNAs 1–7 used in this study. | ||
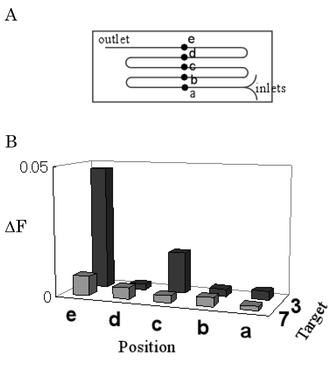 | ||
| Fig. 3 (A) The microchannel structure used in this measurement and (B) detection of sequence-specific binding at any positions. ΔF values are shown as averages (n = 10). This microchannel has a 2 mm diameter curve and 24 cm length. The total flow speed is 40 µl min−1 (1.1 cm s−1). These measurements were carried out at 23 °C. Probe and target DNA solutions are 0.50 pM in 5 mM phosphate buffer (pH 7.0) and 50 mM NaCl. | ||
Fig. 4 shows the results for target DNAs 1–7 at position f in two different temperature and solution conditions. At higher salt concentrations and lower temperature conditions (23 °C, 50 mM NaCl) such as Fig. 4B, ΔF values of targets 1–6, which are complementary and one base mismatch sequences are larger than those of target 7. In contrast, at lower salt concentration and higher temperature conditions (35 °C, 5 mM NaCl) such as in Fig. 4C, ΔF values of short (1) or one base mismatch (5, 6) sequences are relatively low compared to target 7. These results reflect the stability of the double strand in solution. We examined whether target molecule length affects analysis or not. We used 10mer to 70mer DNA as the targets (1, 2, 3, and 4). Fig. 4C indicates that ΔF value increased in proportion to the target molecule length at position f. These results demonstrated that the principle of our strategy can be applied for analysis. We also examined whether this method is applicable for single base mutation analysis or not. Experiments were performed using samples with one base mismatched sequences (5 and 6). Although the experiment was performed as in analysis of a complementary target, ΔF values were almost the same level as in the negative control 7 in Fig. 4C. Thus, we could detect single base mutations; our method is applicable for such SNP analysis. Accuracy of the analysis was also examined. The coefficient of validation for these analyses was nearly 5%. Usually, such a low coefficient of validation cannot be obtained by current technologies such as electrochemical methods utilizing immobilization procedures. In our case, simple syringe pumping might result in such high accuracy. The laminar stream in the microchannel arrives at steady state immediately. At such a steady state, there are no artifacts except for slight pulsation caused by syringe pumping. Therefore our method always offers high accuracy even at small ΔF values.
 | ||
| Fig. 4 (A) The microchannel structure used in this measurement and (B and C) detection of sequence-specific binding. ΔF values are shown as averages (n = 10). The error bars are standard deviations. This microchannel has a 1.5 mm diameter curve and 49 cm length. The total flow speed is 80 µl min−1 (2.2 cm s−1). These measurements were carried out at (B) 23 °C or (C) 35 °C. Probe and target DNA solutions are 0.50 pM in 5 mM phosphate buffer (pH 7.0) and (B) 50 mM or (C) 5 mM NaCl. | ||
Conclusion
We have developed a novel analysis method of sequence-selective DNA detection using microfluidics. This method does not utilize immobilization procedure, but simply uses syringe pumping. By merely injecting target and probe DNA solution, we were able to detect sequence-specific binding of probe and target DNA. The simple operation enables highly accurate DNA analysis: even a single base mismatch could be detected. These features might be suitable for further application of our method in biomedical fields.Acknowledgements
This work was supported by grants from the MEXT of Japan.References
- J. Wang, Chem. Eur. J., 1999, 5, 1681 CrossRef CAS.
- X. Chan, B. Zehnbauer, A. Gnirke and P.-Y. Kwok, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 10756 CrossRef.
- S. Dubiley, E. Kirilov, Y. Lysov and A. Mizabekov, Nucleic Acids Res., 1997, 25, 2259 CrossRef CAS.
- A. C. Pease, D. Solas, E. J. Sullivan, M. T. Cronin, C. P. Holmes and A. P. A. Fodor, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 5022.
- W. M. Howell, M. Jobs, U. Gyllensten and A. J. Brookes, Nature Biotechnol., 1999, 17, 87 CrossRef CAS.
- K. Hashimoto, K. Ito and Y. Ishimori, Anal. Chem., 1994, 66, 3830 CrossRef CAS.
- E. M. Boon, D. M. Ceres, T. G. Drummond, M. G. Hill and J. K. Barton, Nature Biotechnol., 2000, 18, 1096 CrossRef CAS.
- S. Takenaka, K. Yamashita, M. Takagi, Y. Uto and H. Kondo, Anal. Chem., 2000, 72, 1334 CrossRef CAS.
- G. Marrazza, G. Chiti, M. Maschini and M. Anichini, Clin. Chem., 2000, 46, 31 CAS.
- M. Yang, M. E. McGovern and M. Thompson, Anal. Chim. Acta, 1997, 346, 259 CrossRef CAS.
- M. C. Homs, Anal. Lett., 2002, 35, 1875 CAS.
- G. T. A. Kovacs, Micromachined Transducer Sourcebook, McGraw-Hill, 1998 Search PubMed.
- B. H. Weigl and P. Yager, Science, 1999, 283, 346 CrossRef.
- P. J. A. Kenis, R. F. Ismagilov and G. M. Whitesides, Science, 1999, 285, 83 CrossRef CAS.
- H. Kawazumi, A. Tashiro, K. Ogino and H. Maeda, Lab Chip, 2002, 1, 8 RSC.
- M. C. Marin, C. A. Jost, L. A. Brooks, M. S. Irwin, J. O'Nions, J. A. Tidy, N. James, J. M. McGregor, C. A. Harwood, I. G. Yulug, K. H. Vousden, M. J. Allday, B. Gusterson, S. Ikawa, P. W. Hinds, T. Crook and W. G. Kaelin Jr, Nature Genet., 2000, 25, 47 CrossRef CAS.
- G. P. Fasman, CRC Handbook of Biochemistry and Molecular Biology, Nucleic Acids, 1975, CRC Press, 3rd edn., vol. I, 581 Search PubMed.
- J. Sambrook, E. F. Fritsch and T. Maniatis, Molecular Cloning, A Laboratory Manual, Cold Spring Harbor Laboratory Press, 2nd edn., 1989, vol. II, 11.46 Search PubMed.
- J. Kendrew, The Encyclopedia of Molecular Biology, Blackwell Science, 1st edn., 1994, 503 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |

