Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties
Huachang
Lu
ab,
Guangshun
Yi
ac,
Shuying
Zhao
b,
Depu
Chen
*ab,
Liang-Hong
Guo
*b and
Jing
Cheng
bd
aDepartment of Chemistry, Tsinghua University, Beijing, 100084, P. R. China. E-mail: chendp@chem.tsinghua.edu.cn; Fax: (86) 10-62782485; Tel: (86) 10-62781691
bNational Research Engineering Center for Beijing Biochip Technology, 18 Life Science Parkway, Changping District, Beijing, 102206, P. R. China. E-mail: LHGuo@capitalbiochip.com; Fax: (86) 10-80726898; Tel: (86) 10-80726775
cSchool of Materials Science & Engineering, Shandong University of Technology, Zibo, Shandong, 255049, P. R. China
dDepartment of Biological Sciences and Biotechnology, and State Key Laboratory of Biomembrane and Membrane Biotechnology, Tsinghua University, Beijing, 10008, P. R. China
First published on 4th March 2004
Abstract
Multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties were synthesized and characterized. The particles have a core/shell structure. Iron oxide nanoparticles of 5–15 nm diameter were synthesized as the magnetic core. The core was covered with ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er), an efficient infrared-to-visible up-conversion phosphor. The phosphor shell was prepared by co-precipitation of the rare-earth metal salts with fluoride in the presence of EDTA and the magnetic nanoparticle. After the magnetic/fluorescent hybrid particle was coated with SiO2 and activated with glutaraldehyde, streptavidin was immobilized on the particle. The magnetic/fluorescent nanoparticles were found by transmission electron microscopy to be well-dispersed spherical particles with an average diameter of 68 nm. Both energy dispersive X-ray microanalysis and X-ray fluorescence spectra revealed the existence of iron in the particle. Measurements performed on a vibrating sample magnetometer obtained a strong magnetic response for the particle and fluorescence measurements demonstrated its up-conversion property. X-Ray diffraction analysis suggests the phosphor shell has the same structure as the pure NaYF4:Yb,Er nanoparticles we prepared in a previous study (G. S. Yi, H. C. Lu, S. Y. Zhao, Y. Ge, W. J. Yang, L. H. Guo, D. P. Chen and J. Cheng, submitted). Streptavidin-coated magnetic/fluorescent particles were found to bind specifically to a glass slide spotted with biotinylated IgG and emit up-conversion fluorescence, confirming the successful coating of the protein and retention of its optical activity and bio-affinity.
I. Introduction
Magnetic separation and fluorescent labeling are the two most widely used techniques in bioscience. The present work attempts to prepare a new type of nano-sized hybrid particle that combines the two functions of magnetic response and up-converting fluorescence for bio-separation and bio-detection.In the past decade, many new nanoparticle labels have been introduced for biomedical applications. Semiconduction, up-converting, plasmon resonance, magnetism and gold nanoparticles appear to be the most intriguing aspects for in-vitro diagnostics.1–6
The idea of using magnetic techniques in biosciences has enjoyed a resurgence of interest especially during the last two decades. Magnetic adsorbents, carriers and modifiers can be used for the immobilization, isolation, modification, detection, determination and removal of a variety of biologically active compounds, xenobiotics, cellular components and cells. Magnetic separation and labeling have recently found many useful and interesting applications in various areas of biosciences, especially in molecular and cell biology, microbiology, biochemistry and bio-analytical chemistry.7–10
Fluorescent labeling of biological materials using small organic dyes is widely employed in the life sciences, and has been used in a variety of applications that include diagnostics and biological imaging.11 Organic fluorophores, however, have characteristics that limit their effectiveness for such applications. These limitations include narrow excitation bands and broad emission bands with red spectral tails, which can make simultaneous evaluation of several light-emitting probes problematic due to spectral overlaps. Also, many organic dyes exhibit low resistance to photodegradation.12 Up-converting phosphors are inorganic fluorophores that have the potential to circumvent some of the functional limitations encountered by organic dyes in biotechnological applications. They are solid, inorganic, crystalline materials that emit one higher energy photon after absorbing two or more lower energy excitation photons.13 Most recently, there has been a growing interest in using them as signal-generating labels in biological detection.6,14–16 Compared with commonly used organic fluorescent dyes, infrared-to-visible up-converting phosphors have such advantages as low optical background, high photo-stability, and reduced instrument cost. The low background results directly from the large wavelength separation between excitation and emission, as well as the absence of auto fluorescence of biological molecules upon infrared radiation. An enhanced image contrast is expected when these non-fading phosphor particles are applied to detect biomolecules.
To the best of our knowledge, the idea of combining the magnetic response with fluorescence has been reported in only a few articles.17–19 Previous studies on fluorescent magnetic particles mostly concentrated on the preparation and some industrial applications of such compositions. Hatanaka et al. have immobilized FITC-avidin onto the surface of nanoparticles composed of Fe3O4 and γ-Fe2O3 ferrite, and utilized these fluorescent magnetic particles to observe magnetic patterns written on a floppy disk.17 Lu et al. described a sol–gel approach for the coating of superparamagnetic iron oxide nanoparticles with uniform shells of amorphous silica and incorporating fluorescent dyes into the silica shells by covalently coupling these organic compounds with the sol–gel precursor.18 Laurent et al. prepared multifunctional nano-clinics consisting of thin silica-coated magnetic nanoparticles and fluorescent dyes with a bio-targeting group LH-RH. The LH-RH targets receptor-specific cancer cells for utilization in imaging and investigation of biological effects.19 However, there are few reports on the use of fluorescent magnetic hybrid particles as biological materials in bio-separation and bio-labelling. Furthermore, almost all the hybrid particles in previous work used organic dyes as fluorophores, which limit their application.
This paper describes a method of preparing up-converting fluorescent magnetic nanoparticles with covalently coupled streptavidin. As illustrated in Scheme 1, ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er) was deposited on iron oxide nanoparticles by co-precipitation of the rare-earth metal salts in the presence of a chelator, EDTA. The magnetic/fluorescent nanoparticles were then coated with a SiO2 layer formed by the hydrolysis of tetraethyl orthosilicate and 3-aminopropyltrimethoxysilane. After activation with glutaraldehyde, the particles were covalently coupled with streptavidin. Protein arrays were used to confirm the successful binding of streptavidin, and demonstrate one of the possible applications of the multi-functional nanoparticles at the same time. These multifunctional nanoparticles are potentially useful in a variety of areas because they can be simultaneously manipulated with an external magnetic field and characterized in situ using fluorescence microscopy or confocal scanning microscopy. These streptavidin-coated up-conversion fluorescent magnetic nanoparticles can be readily coupled with bio-molecules such as antibodies, proteins and nucleic acids etc.via streptavidin/biotin interaction.
 | ||
| Scheme 1 Procedure for the preparation of streptavidin immobilized up-conversion fluorescent magnetic nanoparticles. | ||
II. Experimental
Materials
Iron(II) sulfate heptahydrate (FeSO4·7H2O), anhydrous ferric chloride (FeCl3), ammonia (NH3·H2O), sodium fluoride (NaF) and disodium ethylenediaminetetraacetic acid (EDTA) were obtained from Beijing Chemical Corporation (Beijing, China). Yttrium oxide (Y2O3, 99.99%), ytterbium oxide (Yb2O3, 99.99%) and erbium oxide (Er2O3, 99.99%) were obtained from Sigma-Aldrich (St. Louis, USA), and were of SpecPure grade. Tetraethyl orthosilicate (TEOS), glutaraldehyde and 3-propanol were obtained from Tianjing Chemical Corporation (Tianjing, China). 3-Aminopropyltrimethoxysilane (APS) and sodium cyanoborohydride were obtained from Acros Organics (New Jersey, USA). Streptavidin was obtained from Promega Corporation (Madison, USA). Biotinylated goat anti-human IgG was obtained from Vector Laboratories (Burlingame, CA) and rabbit anti-human IgG antibody from Cell Marque Corporation (Austin, USA). Aldehyde-modified glass slides were prepared in our lab by following the commonly used silanization procedure.20–22Preparation of superparamagnetic iron oxide nanoparticles
Ferrofluid nanoparticles were prepared by co-precipitation of ferrous and ferric salt with ammonia or sodium hydroxide at room temperature.23 All de-ionized water used in the process was deoxygenated overnight with nitrogen. The reaction was carried out under a nitrogen gas atmosphere.Preparation of up-converting fluorescent magnetic nanoparticles
The ferrofluid nanoparticles prepared above were covered with an up-converting phosphor shell using a co-precipitation method in the presence of rare-earth metal chelator, EDTA.24 In a typical preparation, 2.1 g of NaF (0.05 mol) and 0.100 g superparamagnetic iron oxide nanoparticles were dispersed in 120 mL of de-ionized water by sonication for more than 40 minutes. Another solution was prepared by mixing 16 mL of 0.2 mol L−1 YCl3, 3.4 mL of 0.2 M YbCl3, 0.6 mL of 0.2 mol L−1 ErCl3 and 20 mL of 0.2 mol L−1 EDTA stock solutions together to form the metal–EDTA complexes. The second solution was then injected into the NaF-containing iron oxide suspension quickly, and the mixture was stirred for 1 hour at room temperature. The precipitates from the reaction were separated on a magnetic concentrator and the nonmagnetic particles were washed away with de-ionized water until the wash solution was clear. Then the magnetic composites were heated to 400 °C at a rate of 20 °C per minute, and were kept at this temperature for 5 hours under H2/N2 (5 ∶ 95, v/v) atmosphere. They were then allowed to naturally cool down to room temperature, and the composite of iron oxide and NaYF4:Yb,Er was thus formed.Silica coating and surface treatment with aminosilane
The composites prepared above were coated with silica using the hydrolysis of tetraethyl orthosilicate (TEOS), the famous Stöber process described earlier.20–22 Typically, 30 mg of fluorescent magnetic nanoparticles were dispersed in 80 mL of 3-propanol by sonication for more than 30 minutes. Then 8.94 mL of 28% ammonia (morphological catalyzer), 7.5 mL of de-ionized water (hydrolytic reagent) and 0.1 mL of TEOS were added into the mixture. The mixture was then placed into a constant-temperature oil bath set at 40 °C under vigorous stirring. After 2 hours, 0.1 mL of 3-aminopropyltrimethoxysilane (APS) was added into the suspension and the reaction continued for one more hour. Aminosilane modified magnetic nanoparticles with up-converting fluorescence were thus formed.Streptavidin immobilization
100 mg of aminosilane treated fluorescent magnetic nanoparticles were washed with PBS buffer twice and resuspended in 5 mL of glutaraldehyde solution (glutaraldehyde dissolved in PBS buffer to a final concentration of 12.5% together with 0.1 g of sodium cyanoborohydride), ensuring that the nanoparticles were completely suspended by sonication. It was allowed to react at room temperature for 1∼2 hours, with continuous mixing in order to introduce the aldehyde group. Then the particles were washed with PBS buffer and separated on a magnetic concentrator. 10 mg of glutaraldehyde treated fluorescent magnetic nanoparticles were then resuspended in 4 mL of PBS buffer, 0.6 mg of streptavidin was dissolved in 1 mL of PBS buffer. The two solutions were combined to react at room temperature for 3 hours with continuous mixing. After being washed and isolated from the buffer, the streptavidin-coated particles were resuspended in 5 mL of quenching solution (0.1 mol L−1 Tris and 0.05 mol L−1 sodium cyanoborohydride), and mixed gently for 0.5 h. Finally, the streptavidin coupled fluorescent magnetic nanoparticles were washed and stored at 4 °C in PBS buffer at a concentration of 2 mg mL−1 until used.Detection of streptavidin-biotin interaction on protein array
Biotinylated goat anti-human IgG with a concentration of 1.5 mg mL−1 was spotted manually on an aldehyde glass slide. Rabbit anti-human IgG with a concentration of 1.5 mg mL−1 was spotted in the same array as a negative control. Two arrays were spotted on each glass slide, one was an array of 5 × 5 with one row of negative control and one row of positive control. The positive control used a streptavidin-coated fluorescent magnetic nanoparticle suspension with a concentration of 2 mg mL−1. Another array was of 4 × 4 with one row of negative control and three rows of biotinylated antibody spots (see Fig. 7 later). Spot sizes were 0.9 mm in diameter, spaced 2.0 mm center to center. The spotted samples were allowed to react with the aldehyde groups on the glass slide for 2 h in the air at room temperature. Then the glass slide was put into a Tris solution and reacted for 1 h in order to passivate the active sites on the slide. After quenching, the glass slide reacted with streptavidin-coated fluorescent magnetic nanoparticle suspension (0.2 mg mL−1) for 1 h with vigorous stirring at room temperature. Then the slide was washed with PBST (PBS buffer containing 0.05% Tween 20) buffer twice and deionized water once. Finally the slide was scanned on a home built CCD biochip Scanner with a 980 nm laser at a power of 245 mW.Characterization
The particle size and morphology of all the particulate samples were examined using a Hitachi H-800 transmission electron microscopy (TEM) at accelerating voltages up to 200 kV. The scanning energy for energy dispersive X-ray microanalysis (EDAX, Hitachi H-8010 scanning system, an attachment of TEM) analysis was from 0 to 17 KeV with an elapsed time of 100 s.X-Ray diffraction (XRD) pattern of up-converting fluorescent magnetic nanoparticles were obtained using a D8 advance X-ray diffractometer (Bruker Instruments Inc., Billerica, MA, USA).
The composition of up-conversion fluorescent magnetic nanoparticles was determined semi-quantitatively on an XRF-1700 X-ray fluorescence spectrometer (XRF, Shimadzu Ltd., Tokyo, Japan).
Magnetization measurements of superparamagnetic ferrofluid, up-converting phosphor-coated (UCP-coated) nanoparticles and silica-coated fluorescent magnetic particles were carried out using a vibrating sample magnetometer (VSM, LakeShore 7307, USA) at a field of 5000 G at room temperature.
Fluorescence spectra of the up-conversion fluorescent magnetic nanoparticles were obtained on an LS-50B fluorescence spectrometer (Perkin-Elmer Corp., Forster City, CA, USA) with an external 980 nm laser (260 mW, Beijing Hi-Tech Optoelectronic Corp., Beijing, China) as the excitation source in place of the xenon lamp in the spectrometer.
The up-converting fluorescence images of fluorescent magnetic nanoparticles were obtained using a combined system with a motorized inverted fluorescence microscope, an external 980 nm laser (260 mW, Beijing Hi-Tech Optoelectronic Corp., Beijing, China) as the excitation source and a CCD camera. An external magnetic field was generated with a rare earth alloy magnet (ProMega Corp.).
III. Results and discussion
Morphology and structure of the nanoparticles
The overall process of preparing up-converting fluorescent magnetic nanoparticles is represented in Scheme 1. The emphasis of the work was on the coating of the superparamagnetic particles with up-converting phosphors and the coating of these composites with a silica shell. The superparamagnetic nanoparticles were prepared following the published procedure.23 Transmission electron microscopy (TEM) of superparamagnetic nanoparticles shows that the iron oxide particles dispersed in water have dimensions in the range of 5–15 nm (Fig. 1a). The size distribution seems to be wider than those prepared in the literature,23 one likely reason being that we did not use any surfactant in the synthesis. Also, our procedure is not yet fully optimized to produce mono-dispersed nanoparticles. Fig. 1b shows that the UCP-coated magnetic nanoparticles are fairly well dispersed, nearly spherical and with a relatively narrow size distribution. The histogram of the particle diameter (Fig. 1e) reveals that most of the particles illustrated in Fig. 1b are 53–87 nm in diameter, with an average value of about 68 nm. Figs. 1c and 1d present images of up-conversion fluorescent magnetic nanoparticles after coating with a silica layer. It is observed that the particle has a core-shell structure and the silica shell is clearly visible. The silica-coated hybrid nanoparticles have a particles size ranging from 80 to 150 nm and a regular spherical shape present in most of the samples. It is also observed that, in some cases, the particles are co-encapsulated by the silica shell into aggregates, which would increase the polydispersity of the particles after silica treatment. However, the silica shell has little effect on the properties of magnetism and fluorescence, and does not affect their use in bioassays, as shown later.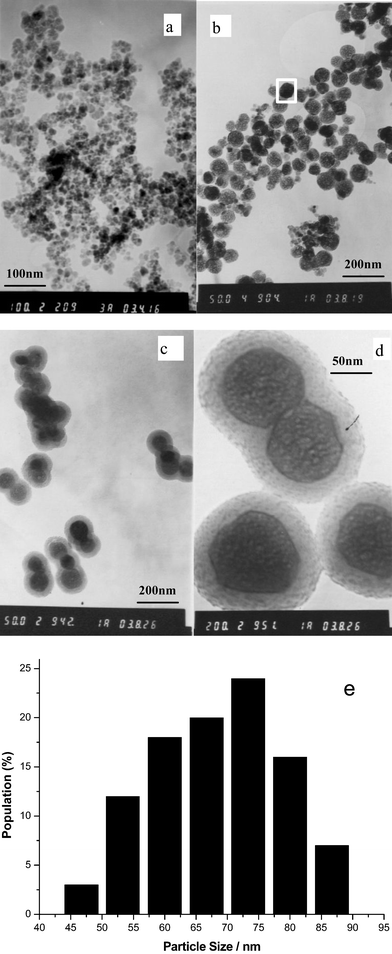 | ||
| Fig. 1 Transmission electron micrographs of (a) superparamagnetic iron oxide nanoparticles; (b) UCP-coated magnetic nanoparticles; (c, d) up-conversion fluorescent magnetic nanoparticles whose surfaces have been coated with silica shells and (e) size distribution of (b). | ||
The silica shell thickness is about 20 to 30 nm and could vary with the dosage of tetraethyl orthosilicate (TEOS) and the reaction time.21,22 In our previous work,24 ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er) nanoparticles were synthesized via a co-precipitation method. The multifunctional nanoparticles prepared in this work are composites of magnetic iron oxides and ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er) phosphors. In order to investigate the structure and composition of the hybrid nanoparticles, XRD, EDAX and XRF were employed to analyze the sample of UCP-coated nanoparticles. X-Ray diffraction analysis shows that the composites have nearly the same pattern as ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er) nanoparticles (Fig. 2). This indicates that the up-converting phosphors in the hybrid nanoparticles have the same structural basis of NaYF4:Yb,Er, which has a cubic (ICDD No. 77-2042, marked with a ☆ in Fig. 2) and a hexagonal phase (ICDD No. 28-1192, also called α-NaYF4:Yb,Er, marked with a ○ in Fig. 2). They are the materials which emit up-conversion fluorescence when excited with a 980 nm laser.
 | ||
| Fig. 2 X-Ray diffraction spectra of ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4:Yb,Er) phosphor powder sample (A) and up-conversion fluorescent magnetic nanoparticle powder sample (B). The diffraction peaks marked with stars (☆) are indexed for a cubic phase for NaYF4:Yb,Er and the peaks marked with circles (○) are indexed for a hexagonal phase for NaYF4:Yb,Er. | ||
Probably because magnetic materials in the composites are of low content or low level of crystallization, no distinct peaks appear on the XRD pattern. However, elemental analysis by EDAX established that the fluorescent magnetic nanoparticles contained Fe (Fig. 3). To further substantiate our claim for the presence of a magnetic component, the composition of the hybrid nanoparticles was determined semi-quantitatively by XRF. This result showed that the molar ratio of Y ∶ Fe is 1 ∶ 0.126 in the composite nanoparticles. The vibrating sample magnetometer (VSM) result discussed below also revealed the presence of magnetic materials.
 | ||
| Fig. 3 EDAX spectrum of up-conversion fluorescent magnetic nanoparticles. The scanning area is marked with a white rectangle in Fig. 1b. | ||
In order to use these multifunctional nanoparticles in bio-separation and bio-detection, surface coating and surface modification was applied to them. After coating with silica, it is possible to introduce functionality such as amino groups onto the surface of particles through the hydrolysis of 3-aminopropyltrimethoxysilane (APS).
Demonstration of multifunctionality of the fluorescent magnetic nanoparticles
The magnetic and up-conversion optical properties of the multifunctional nanoparticles were studied with VSM, fluorescence spectroscopy and fluorescence microscopy with an external 980 nm laser. Magnetic hysteresis loops, as shown in Fig. 4, were measured on powder samples of ferrofluids, uncoated and silica-coated hybrid nanoparticles at room temperature. The saturation magnetization values of silica-coated and uncoated magnetic up-converting particles are 5.3 emu g−1 and 8.4 emu g−1 respectively. These numbers are much lower than that of ferrofluid iron oxide (about 65 emu g−1, Fig. 4), probably because of the thick shells coated on magnetic nanoparticles. Nonetheless, the magnetization values are enough for common bio-separation. The saturation magnetization value can be adjusted through control of the shell thickness. The ferrofluids with an average diameter of less than 15 nm prepared in the present work are superparamagnetic because of the nearly zero coercivity. In previous studies, high-resolution TEM indicates that there are probably two types of iron oxide particles in ferrofluids: maghemite (γ-Fe2O3) and magnetite (Fe3O4).25 The coexistence of maghemite and magnetite could be attributed to oxidation of Fe3O4 to γ-Fe2O3 during the synthesis.26–28 Both types of nanoparticles have high magnetism and nearly zero coercivity, and therefore can be used as cores of the hybrid nanoparticles. But the superparamagnetism disappeared after coating, and ferromagnetic response was found because of the non-zero coercivity of these hybrid nanoparticles (about 38 G). The switch from superparamagnetic to ferromagnetic behavior for these nanoparticles occurred at ∼13 nm, which was already reported in previous studies.29,30 The data suggest that in the coated hybrid particle the core consists of ferrofluid nanoparticles larger than their original size of 5–15 nm, possibly due to aggregation during annealing of the up-conversion phosphor coating.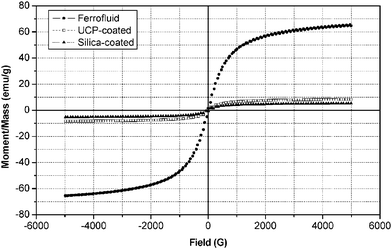 | ||
| Fig. 4 Magnetization curves of ferrofluids (●), UCP-coated magnetic nanoparticles (□), and silica-coated fluorescent magnetic nanoparticles (▲). | ||
After the magnetic nanoparticle was coated with NaYF4:Yb,Er, green and red up-conversion emissions were observed with 980 nm infrared excitation. Fig. 5 shows the infrared-to-visible up-conversion fluorescence spectrum of the sample. The two emission peaks at 539 and 658 nm, assigned respectively to 4S3/2 to 4I15/2 and 4F9/2 to 4I15/2 transition of erbium,31–33 are similar to the emission peaks observed in our previous report for the pure up-conversion nano-particle itself. Therefore the up-conversion fluorescence property was preserved when the material was coated as a shell on magnetic nano-particles. These hybrid nanoparticles with up-conversion fluorescence have many optical advantages (e.g. low optical background, high photo-stability and low laser cost) and thus can be employed as reporters in immunoassays or DNA assays.
 | ||
| Fig. 5 Infrared-to-visible up-conversion fluorescence spectrum of UCP-coated magnetic nanoparticles with 980 nm excitation after annealing at 400 °C for 5 h. | ||
The activity and properties of the hybrid nanoparticles under a fluorescence microscope may better illustrate their multi-functionality. Fig. 6a shows the up-conversion fluorescence image of UCP-coated magnetic nanoparticles in the presence of a magnetic field generated with a rare earth alloy magnet. The samples were excited with a 980 nm laser. These hybrid nanoparticles lined up to form a chain-like structure (with their longitudinal directions oriented along the magnetic field) because of magnetization of each aggregate particle. Fig. 6b shows the up-conversion fluorescence image of a stack-like structure formed by the UCP-coated magnetic nanoparticles under the influence of a needle-like magnet. The images demonstrate the magnetic and optical properties of the hybrid particles. The inset is a TEM image of the corresponding nanoparticles (deposited on TEM grids under no magnetic field), showing the dispersed state of the particle.
 | ||
| Fig. 6 Fluorescent microscopy images of UCP-coated magnetic nanoparticles excited with a 980 nm laser; (a) image of chain-like structures formed by UCP-coated magnetic nanoparticles in the presence of an external flat magnetic field; (b) image of stack-like structures formed by the same nanoparticles in an external needle-like magnetic field. | ||
Streptavidin immobilization and detection on a protein chip
The surface of the magnetic/fluorescent hybrid nanoparticles coated with silica was modified sequentially with aminosilane and glutaraldehyde, which reacts readily with primary amines on proteins to form a Schiff base linkage. Immobilization of proteins onto functionalized particles with maximum retention of activity and minimum non-specific interaction is a key goal in the development of many diagnostic, affinity separation, and biomaterial technologies. One of the widely used proteins in current diagnostic and separations technologies is streptavidin, and there has been considerable use of immobilized streptavidin for both commercial and laboratory purposes. In the present work streptavidin was immobilized on the aldehyde-activated hybrid particles. Once prepared, the streptavidin-coated particles can be further coated with other biotinylated biomolecules. The success of streptavidin immobilization on the hybrid nano-particle was demonstrated by its specific binding with an array of biotinylated IgG spots on a glass slide. The protein array is illustrated in Fig. 7. Row A in array (a) and row H in array (b) were spotted with rabbit anti-human IgG as negative control. Row B in array (a) was spotted with streptavidin-coated hybrid particles as positive control. The biotinalyted goat anti-human IgG was spotted on the other rows to recognize and bind streptavidin-coated hybrid particles dispersed in solution. A home-built CCD biochip scanner with an external 980 nm laser and an infrared filter was used to detect the infrared-to-visible up-conversion fluorescence on the glass slide. Signal from the positive control spots assured the working state of the glass slide and scanner, whereas the absence of any signal from the negative control spots illustrated that non-specific absorption was low. Therefore, up-conversion fluorescence signal from the biotinylated IgG spots came from strepavidin-coated hybrid nanoparticles bound to the glass slide through the specific biotin/streptavidin interaction. The results confirmed that streptavidin was coated successfully on the particle. | ||
| Fig. 7 Schematic of the protein assay on an aldehyde group-modified glass slide for the detection of specific immobilization of streptavidin and the CCD images of the two arrays (a) and (b). Rows C, D, E, F, G and I were spotted with biotinylated goat anti-human IgG (1.5 mg mL−1) as specific biotinylated reagent; rows A and H were spotted with rabbit anti-human IgG (1.5 mg mL−1) as negative control; row B was spotted with streptavidin-coated hybrid particles (3 mg mL−1) as positive control. The slide reacted with streptavidin-coated hybrid nanoparticles suspension (0.2 mg mL−1). | ||
IV. Conclusions
Hybrid nanoparticles possessing magnetic response, up-conversion fluorescence and bio-affinity were prepared successfully by co-precipitation of NaYF4:Yb,Er up-converting phosphors on superparamagnetic nanoparticles and subsequently coated with SiO2 and immobilized with streptavidin. TEM reveals the well-dispersed spherical shape and core-shell structure of the nanoparticles. XRD spectra show the crystalline nature of NaYF4:Yb,Er up-converting phosphors in the hybrid particles. EDAX and XRF reveal the ferric components of the composites. The multi-functionality of the nanoparticles was demonstrated by VSM, fluorescence spectroscopy, fluorescence microscopy and bio-affinity reaction. These results show that the hybrid nanoparticles have magnetic response and red/green up-conversion fluorescence. The protein arrays reveal the successful surface immobilization of streptavidin. The streptavidin-coated fluorescent magnetic nanoparticles can be used for immobilization of biotinylated biomolecules and subsequent capture of target molecules in samples. They can then be manipulated with a magnetic field for separation or purification, and characterized in situ using conventional fluorescence microscopy or a fluorescence spectrometer.V. Acknowledgements
We are grateful to Professors Y. D. Li and X. R. Zhang for helpful discussion and advice. Financial support from the National High-Tech Program (No. 2002AA2Z2011) and National Key Basic Research Development Program (G19990116) is gratefully acknowledged.VI. References
- M. J. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 28, 2013 CrossRef.
- W. C. W. Chan and S. M. Nie, Science, 1998, 281, 2016 CrossRef CAS.
- S. Schultz, D. R. Smith, J. J. Mock and D. A. Schultz, Proc. Natl. Acad. Sci. U. S. A., 2000, 97(3), 996 CrossRef CAS.
- Y. R. Chemla, H. L. Grossman, Y. Poon, R. McDermott, R. Stevens, M. D. Alper and J. Clarke, Proc. Natl. Acad. Sci. U. S. A., 2000, 97(26), 14268 CrossRef CAS.
- T. A. Taton, C. A. Mirkin and R. L. Letsinger, Science, 2000, 289, 1757 CrossRef CAS.
- J. Hampl, M. Hall, N. A. Mufti, Y. M. M. Yao, D. B. MacQueen, W. H. Wright and D. E. Cooper, Anal. Biochem., 2001, 288, 176 CrossRef CAS.
- M. Safarikova and I. Safarik, Magn. Electr. Sep., 2001, 10, 223 Search PubMed.
- Q. A. Pankhurst, J. Connolly, S. K. Jones and J. Dobson, J. Phys. D: Appl. Phys., 2003, 36, 167 CrossRef.
- P. Tartaj, M. D. P. Morales, S. Veintemillas-Verdaguer, T. Gonzalez-Carreno and C. J. Serna, J. Phys. D: Appl. Phys., 2003, 36, 182 CrossRef.
- C. C. Berry and A. S. G. Curtis, J. Phys. D: Appl. Phys., 2003, 36, 198 CrossRef.
- E. Schröck, S. du Manoir, T. Veldman, B. Schoell, J. Wienberg, M. A. Ferguson-Smith, Y. Ning, D. H. Ledbetter, I. Bar-Am, D. Soenksen, Y. Garini and T. Ried, Science, 1996, 273, 494 CAS.
- G. T. Hermanson, Bioconjugate Techniques, Academic Press, London, UK, 1996, ch. 8 Search PubMed.
- A. C. Tropper, J. N. Carter, R. D. T. Lauder, D. C. Hanna, S. T. Davey and D. Szebesta, J. Opt. Soc. Am., 1994, 11, 886 Search PubMed.
- P. Corstjens, M. Zuiderwijk, A. Brink, S. Li, H. Feindt, R. S. Neidbala and H. Tanke, Clin. Chem., 2001, 47, 1885 CAS.
- P. L. A. M. Corstjens, M. Zuiderwijk, M. Nilsson, H. Feindt, R. S. Niedbala and H. J. Tanke, Anal. Biochem., 2003, 312, 191 CrossRef CAS.
- F. van de Rijke, H. Zijlmans, S. Li, T. Vail, A. K. Raap, R. S. Niedbala and H. J. Tanke, Nat. Biotechnol., 2001, 19, 273 CrossRef.
- S. Hatanaka, N. Matsushita, M. Abe, K. Nishimura, M. Hasegawa and H. Handa, J. Appl. Phys., 2003, 93, 10 CrossRef.
- Y. Lu, Y. D. Yin, T. Mayers Brian and Y. N. Xia, Nano Lett., 2002, Vol 2, 183 CrossRef CAS.
- L. Laurent, S. Yudhisthira, K. S. Kim, E. J. Bergey and P. N. Prasad, Chem. Mater., 2002, 14, 3715 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- M. Ohmori and E. Matijevic, J. Colloid Interface Sci., 1992, 150, 594 CrossRef CAS.
- M. Ohmori and E. Matijevic, J. Colloid Interface Sci., 1993, 160, 288 CrossRef CAS.
- Y. S. Kang, S. Risbud, J. F. Rabolt and P. Stroeve, Chem. Mater., 1996, 8, 2209 CrossRef CAS.
- G. S. Yi, H. C. Lu, S. Y. Zhao, Y. Ge, W. J. Yang, L. H. Guo, D. P. Chen and J. Cheng, submitted.
- S. A. Iakovenko, A. S. Trifonov, M. Giersig, A. Manedov, D. K. Nagesha, V. V. Hanin, E. C. Soldatov and N. A. Kotov, Adv. Mater., 1999, 11, 388 CrossRef CAS.
- R. E. Vandenberghe, R. Vandenberghe, E. De Graveand and G. Robbrecht, J. Magn. Magn. Mater., 1980, 15, 1117 CrossRef.
- T. Sato, T. Iijima, M. Seki and J. Inagaki, J. Magn. Magn. Mater., 1987, 65, 252 CrossRef CAS.
- E. Tronc, P. Belleville, J. P. Jolivet and J. Livage, Langmuir, 1992, 8, 313 CrossRef CAS.
- K. A. Eason, K. J. Klabunde, C. M. Sorensen and G. C. Hadjipanayis, Polyhedron, 1994, 13, 1197 CrossRef CAS.
- C. P. Bean and J. D. Livingston, J. Appl. Phys., 1959, 30, 120S CAS.
- M. A. Chamarro and R. Cases, J. Luminesc., 1990, 46, 59 Search PubMed.
- M. Shojiya, M. Takahashi, R. Kanno, Y. Kawamoto and K. Kadono, Appl. Phys. Lett., 1994, 65, 1874 CrossRef CAS.
- T. Hirai, T. Orikoshi and I. Komasawa, Chem. Mater., 2002, 14, 3576 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
