Metallomics as integrated biometal science
Hiroki
Haraguchi
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan
First published on 26th November 2003
Abstract
In this paper, “metallomics” is proposed as a new scientific field in order to integrate the research fields related to biometals. Metallomics should be a scientific field in symbiosis with genomics and proteomics, because syntheses and metabolic functions of genes (DNA and RNA) and proteins cannot be performed without the aid of various metal ions and metalloenzymes. In metallomics, metalloproteins, metalloenzymes and other metal- containing biomolecules are defined as “metallomes”, in a similar manner to genomes in genomics as well as proteomes in proteomics. Since the identification of metallomes and the elucidation of their biological or physiological functions in the biological systems is the main research target of metallomics, chemical speciation for specific identification of bioactive metallomes is one of the most important analytical technologies to establish metallomics as the integrated bio-metal science. In order to rationalize the concept of metallomics, the distributions of the elements in man, human blood serum and sea-water, a challenge to all-elements analysis of one biological cell, and some other research topics are introduced with emphasis on recent development of chemical speciation of trace metals in some biological samples.
1. Introduction
In trace analysis, the total concentrations of the elements in various biological or environmental samples have been usually determined by the highly-sensitive analytical methods such as AAS (atomic absorption spectrometry), ICP-AES (inductively coupled plasma atomic emission spectrometry), ICP-MS (inductively coupled plasma mass spectrometry), XRF (X-ray fluorescence spectrometry), NAA (neutron activation analysis) and so on.1,2 Since hazardous or toxic elements, such as Hg, Cd, Pb, Cr(VI), As, Sn and Se, have caused serious environmental pollution or toxicological problems, such elements in the biological, environmental, and geochemical samples have been extensively determined for environmental management and/or protection from hazardous infections.On the other hand, it has been elucidated that various elements play essential roles in various biological systems.3–9 In fact, most of trace metals in biological fluids and organs are binding with various proteins, which are called “metalloproteins”. Metalloproteins are called “metalloenzymes” when they work as the biological catalysts to regulate the biological reactions and physiological functions in biological cells and organs. Some typical metalloenzymes and metalloproteins are summarized in Table 1. As is seen in Table 1, metalloenzymes contain the specific number of metal ion(s) at the active sites in specific proteins,7–9 and they work as biocatalysts for specific enzymatic reactions including gene (DNA, RNA) synthesis, metabolism, antioxidation and so forth. In addition, the bioavailability and toxicity of the elements also depend on their chemical forms. Thus, species analyses of metal-binding molecules in biological samples are an important subject in various scientific fields such as biochemistry, biology, medicine, pharmacy, nutrition, agriculture, environmental science and so on. Accordingly, in recent years, chemical speciation or elemental speciation has been extensively developed to elucidate the biological essentiality and toxicity of the elements on the molecular basis.1,2,10–17
| Metalloenzyme (MWa/kDa) | Number of atom | Biological function |
|---|---|---|
| a Molecular weight. | ||
| Transferrin (66–68) | 2Fe | Transportation of iron |
| Ferritin (473) | 1Fe | Storage of iron |
| Catarase (225) | 4Fe | Decomposition of H2O2 |
| Nitrogenase (200–220) | 24Fe, 2Mo | Nitrogen fixation |
| Chitochrome P-450 (50) | 1Fe | Metabolisms of steroids and drugs |
| Carbonic anhydrase (30) | 1Zn | Catalyst of H2CO3 equilibrium (H2CO3 ⇄ CO2 + H2O) |
| Caboxypeptidase (34) | 1Zn | Hydrolysis of peptide bonds at carboxyl terminal |
| Alcohol dehydogenase (150) | 4Zn | Dehydration of alcohol (C2H5OH ⇄ CH3CHO + H2) |
| Alkaline phosphatase (89) | 3.5Zn | Hydrolysis of phosphate esters |
| DNA polymerase (109) | 2Zn | DNA synthesis |
| RNA polymerase (370) | 2Zn | RNA synthesis |
| Plastocyaneine (134) | 1Cu | Electron transfer |
| Gluthathionperoxidase (76–92) | 1Se | Decomposition of H2O2 and organic superoxides |
| Urease (480) | 10Ni | Transformation of urease to ammonia |
Another example of progress in analytical methods such as ICP-AES and ICP-MS is the multielement detection capability with high sensitivity and wide linear dynamic range for most metallic and metalloid elements.1,2,18,19 Such analytical features as ICP-AES and ICP-MS enable us to determine almost all elements in the major-to-ultratrace concentration ranges. Taking into consideration recent state-of-art developments in analytical atomic spectrometry, in 1998 the present author published a review paper entitled Multielement profiling analysis of biological, geochemical and environmental samples as studied by analytical atomic spectrometry,1 in which the importance of multielement data for various samples was emphasized with the concept of the Extended All Present Theory of the Elements, proposed by the present author in 1994,1,20–22 which is a concept that all elements in the periodic table are supposed to be contained not only in all geochemical samples, but also in the biological samples from any kind of biological species including man. Thus, the Extended All Present Theory of the Elements is a wider concept than the All Present Theory of Noddack, which is only for geochemical samples (rocks and minerals), proposed in 1932.23,24 The extreme goal of the Extended All Present Theory of the Elements should be to verify the existence of all elements in one biological cell.1
Against this research background, the present author proposed the concept and terms “metallomics” and “metallomes” for integrated bio-trace element science25 at the International Symposium on Bio-trace Elements 2002 (BITREL 2002), held in October, 2002, under the sponsorship of the Institute of Physical and Chemical Research, Japan. In 2001, Williams used the term “metallome” in his review paper26 entitled Chemical selection of elements by cells, in which he mentioned that “The variety of paths which individual elements follow in any organ adds to the specific character of the organisms. Clearly the paths have evolved to create an elemental distribution which we shall call the metallome, to parallel the nomenclature of protein distribution, the proteome.” Recently, in Science,27 the special section “Metals: Impacts on Health and the Environment” was published, where several topics on metal ions in the environment and health in relation to chemistry, medicine, ecology and oceanography were described with emphasis on health and disease. In the special section, Ash and Stone used the term of “metallome” in the following sentence:28 “In the world of the ‘metallome’, it is indeed a narrow path between poison and nutrition.” However, any definition or explanation of “metallome” was not given there. In the two review papers introduced above, the term “metallome” was used rather to explain the elemental distribution and selection of metals in biological cells and organs, and the functional aspects of bio-metals was never emphasized to explore the systematic research field concerning biometal science in future.
Hence, in the present paper, further consideration of metallomics is described to establish the concept and to promote scientific research for biometal science from the viewpoint of “metal-assisted function bioscience (or biochemistry)”.
2. Metallomics and metallomes
In order to gain an insight into the scientific field of “metallomics”, a schematic model of the biological system is illustrated in Fig. 1, where a biological cell and biological fluid (e.g., blood serum) are separated by the cell membrane. On the left hand side, academic terms such as genomics, proteomics and metabollomics are shown along with metallomics to indicate their research areas in the biological system. As is well known, genomics deals with the scientific works on the genetic information of DNAs and RNAs encoded as the sequences of nucleic bases. Such DNAs and RNAs are generally called genomes in genomics, which play essential roles of protein syntheses. Proteins are distributed inside and outside the cell, and they work as enzymes for synthesis and metabolism of various biological substances inside the cell. It is seen from Table 1 that DNAs and RNAs are synthesized by DNA polymerases and RNA polymerases, which are zinc enzymes. Since a large number of proteins play essential roles in syntheses and metabolisms of many biological molecules to regulate and maintain the life system, protein science has been receiving great attention as post-genome science linked with genomics.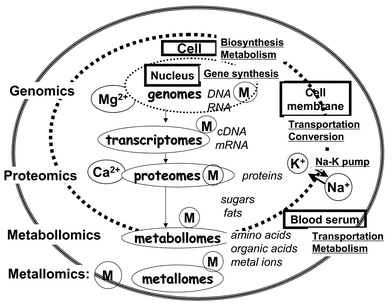 | ||
| Fig. 1 A schematic model of the biological system, showing the relationships among genomics, proteomics, metabollomics and metallomics, where metallic ions are shown as M. | ||
Many biological substances as well as metal ions are transported as raw materials inside the cell through the membrane. In general, material conversion is actively occurring inside the cell and also often in the cell membrane, and such material conversion and transportation processes out of the cell are called “metabolism”. Biological substances, which are usually small molecules such as amino acids, organic acids and metal ions produced in metabolism, have recently been called “metabollomes” or “metabolites”.29
Bio-sciences concerned with metallic elements and their applied sciences have been studied independently in many scientific fields such as biochemistry, bioinorganic chemistry, nutritional science, pharmacy, medicine, toxicology, agriculture, environmental science and so forth. All such scientific fields have a deep interrelationship, with the common factor of “metals”, from the viewpoint of biological science. Therefore, it is desirable that biometal science is promoted as the interdisciplinary field to integrate the metal-related scientific fields. Thus, the present author is newly proposing the academic nomenclature of “metallomics” for biometal science. In the study of metallomics, elucidation of the physiological roles and functions of biomolecules binding with metallic ions in the biological systems should be the most important research target. Then, metallomics may be called, in another words, “metal-assisted function biochemistry”.
In recent years, genomics and proteomics have received great attention to appreciate various biological systems from the viewpoints of gene and protein sciences. Genomics and proteomics are indeed the fundamentally important scientific fields, because genes (DNAs and RNAs) contain the genetic information codes to synthesize various proteins. However, as is seen from Table 1, genes and proteins cannot be synthesized without the assistance of metalloenzymes containing zinc and other metals. In these senses, metallomics may stand in the same position in scientific significance as genomics and proteomics. Thus, in metallomics, biological molecules bound with biometals are properly defined as “metallomes”, corresponding to genomes and proteomes in genomics and proteomics, respectively. However, metallic ions such as alkali and alkaline earth metal ions, which exist mostly as free ions in biological fluids, should also be included in metallomes because they also play many important roles in the occurrence of the physiological functions in the biological systems.
As mentioned above, various scientific fields related to biometals have been independently established so far, but from now on it will be desirable to integrate such scientific fields under the term “metallomics” with emphasis on the identification of biomolecules bound with metal ions as well as on their functions in the biological systems. From the above consideration, then, the main research subjects in metallomics may be summarized as in Table 2. In the study on the subjects in metallomics, listed in Table 2, many advanced analytical techniques are needed to explore the new research fields for integrated biometal science. Some of such analytical techniques are shown in Table 2. So far, the hyphenated techniques such as LC-ICP-MS, GC-ICP-MS and LC-ICP-AES have been developed as the analytical methods for chemical speciation of trace metals in the biological samples.1,11–15 Such hyphenated methods are actually the most powerful techniques for chemical speciation. However, they are useful only for the identification of known and stable compounds such as methylmercury, methylated arsenics, butyltin compounds and so on.2,11–15,30,31 From now on, however, it is obvious that the identification of biomolecules, such as metalloproteins and metalloenzymes, as well as metal-binding nucleic acids and metabolites, will become more important in exploring biometal science in relation to their biological functions and metabolism. Then, the methods for the direct identification of biomolecules, such as ES-MS (electrospray mass spectrometry) and MALDI-TOFMS (matrix-assisted laser desorption ionization time-of-flight mass spectrometry), should be employed for the study on chemical speciation.13,32–35 Maybe, a system of LC doubly combined with ES-MS (or MALDI-TOFMS) and ICP-MS, i.e., LC-ES-MS-ICP-MS, which is a kind of tandem mass spectrometry13 and allows us to detect organic molecules and trace metals simultaneously, may be the more ideal instrumentation for direct speciation of unknown organometallic compounds and metalloproteins to promote development and establishment of metallomics. It is noted here that presently the detection sensitivities for organic molecules obtained by organic mass spectrometry seem to be inferior to those for trace metals by ICP-MS. Therefore, the sensitivity-matching in the hyphenated system, for example, using ES-MS and ICP-MS, should be explored by improving the ionization efficiencies of bio-organic molecules in organic mass spectrometry to develop such a simultaneous detection system.
| Research subject | Analytical technique |
|---|---|
| a Atmospheric pressure chemical ionization mass spectrometry. | |
| 1. Distributions of the elements in the biological fluids, cell, organs, etc. | Ultratrace analysis, all-elements analysis, one atom detection, one molecule detection |
| 2. Chemical speciation of the elements in the biological samples and systems | Hyphenated methods (LC-ICP-MS, GC-ICP-MS, MALDI-MS, ES-MS) |
| 3. Structural analysis of metallomes (metal-binding molecules) | X-ray diffraction analysis, EXAFS |
| 4. Elucidation of reaction mechanisms of metallomes using model complexes (bioinorganic chemistry) | NMR, XPS, laser-Raman spectroscopy, DNA sequencer, amino acids sequencer, time- resolution and spatial-resolution fluorescence detection |
| 5. Identification of metalloproteins and metalloenzymes | LC-ES-MS, LC-MALDI-MS, LC-ICP-MS |
| 6. Metabolisms of biological molecules and metals (metabollomes, metabolites) | LC, GC, LC-MS, GC-MS, ES-MS, API-MSa, biosensors |
| 7. Medical diagnosis of health and disease related to trace metals on a multielement basis | ICP-AES, ICP-MS, graphite-furnace AAS, autoanalyser, spectrophotometry |
| 8. Design of inorganic drugs for chemotherapy | LC-MS, LC-ICP-MS, stable isotope tracers |
| 9. Chemical evolution of the living systems and organisms on the earth | Isotope ratio measurement (chronological techniques), DNA sequencer |
| 10. Other metal-assisted function biosciences in medicine, environmental science, food science, agriculture, toxicology, biogeochemistry, etc. | In-situ analysis, immunoassay, bio-assay, food analysis, clinical analysis |
3. Distributions of major-to-ultratrace elements in man, human blood serum and sea-water
As is listed in Table 2, study of the distributions of major-to-ultratrace elements in biological and biogeochemical samples is one of main research subjects in metallomics, because such information of the distributions may provide primarily important information about health and disease in medical diagnosis. In addition, if we can know the elemental distributions in the cells or organs of every organism, such information about the distributions of the elements may be available for medical diagnosis of health and disease as well as drug design for chemotherapy, together with information about the specific functions of metal-binding biomolecules. Thus, it is desirable to realize all-elements analysis of one biological cell to obtain metal-fingerprints for the biological species, similar to genetic codes in genes and the amino acids-sequence in proteins.In Table 3,36 the average amounts of major-, minor-, trace- and ultratrace elements contained in a human body (70 kg weight) are summarized together with their biological functions and diseases, where bio-essential trace elements proved for man and experimental mammals are marked with * and **, respectively. It is seen from Table 4 that many elements are universally contained in our human body at the different concentration levels, and that most of them play essential roles as metalloproteins and metalloenzymes in the blood and organs of biological systems. Furthermore, the antioxidation functions of Se (glutathioneperoxidase) and Fe (catarase) are important to protect the biological systems from oxidation caused by peroxides. Superoxide dismutases, which usually contain Mn, Fe, Cu and/or Zn, act as antioxidants against superoxides. In addition, it is known that Na and K act as Na–K pumps at the cell level to perform material transportation through cell membranes, which is promoted by their concentration gradients inside and outside the membranes. Although the functions of metallic elements cannot be explained in more detail here, there is no doubt that man and any other biological species cannot maintain their life systems without the aid of a variety of metallic elements. Thus, biometal science such as metallomics should be systematically developed to appreciate the whole biological systems as well as to identify molecules and their biological functions for syntheses and metabolisms.
| Elementb | Total amount (70 kg weight) | Functions and diseases | |
|---|---|---|---|
| a Cited from ref. 36 with some revision. b *; Essential elements in man, **; Essential elements in experimental mammals. | |||
| Major elements | O | 45.5 kg | Compositions of water and organics |
| C | 12.6 | Compositions of organics | |
| H | 7.0 | Compositions of water and organics | |
| N | 2.1 | Compositional elements of amino acids, proteins, nucleic acids and so on | |
| Ca | 1.05 | Main constituents of bones, compositions of cell membrane, blood coagulation factors | |
| P | 0.70 (98.5%) | Main constituents of bones, compositional elements of nucleic acids and ATP | |
| Minor elements | S | 175 g | Compositions of amino acids (cysteine, methionein), Vitamin B1, biotin, heparin, etc. |
| K | 140 | Compositions of inner-cell electrolytes | |
| Na | 105 | Compositions of outer-cell electrolytes | |
| Cl | 105 | Main anions existing inside and outside cells | |
| Mg | 105 (99.4%) | Co-factors of enzymes, composition of chlorophylls | |
| Trace elements | Fe* | 6 | Transportation and storage of oxygen, transportation and storage of iron, antioxidation |
| F** | 3 | Concomitant elements in bone | |
| Si* | 2 | Unknown | |
| Zn* | 2 | Co-factors of enzymes, cell division, nucleic-acids polymerases | |
| Sr | 32 mg | Unknown | |
| Rb** | 320 | Unknown | |
| Pb** | 120 | Metabolism of iron, blood making | |
| Mn* | 100 | Co-factors (superoxide dismutase, pyruvate kinase), metabolism of fats | |
| Cu* | 80 | Transportation of oxygen, redox reactions, electron transfer, oxygen addition | |
| Ultratrace elements | Al | 60 | Alzheimer disease |
| Cd | 50 | Auch-auch disease | |
| Sn** | 20 | Redox reactions | |
| Ba | 17 | Unknown | |
| Hg | 13 | Minamata disease (methylmercury) | |
| Se* | 12 | Antioxidation (glutathione peroxyalase), mercury toxicity reduction | |
| I* | 11 | Activation of thyroid gland | |
| Mo* | 10 | Co-factors of enzymes (xythanthin oxydase, nitrate reductase), metabolism of uric acid | |
| Ni** | 10 | Co-factor of enzyme (urease), stabilization of RNAs | |
| Cr* | 2 | Glucose tolerance factor, metabolisms of fats and proteins | |
| As** | 2 | Metabolic activation of zinc | |
| Co* | 1.5 | Blood making (vitamin B12) | |
| V** | 1.5 | Metabolism of cholesterols, inhibitors of Na+- and K+-ATPase | |
| Elementa | Concentration/µg ml−1 | ||
|---|---|---|---|
| Blood serumb [A] | Sea-waterc [B] | Ratio [A]/[B] | |
| a The elements with no mark, † or ††, are lithophile, siderophile and chalcophile elements, according to the geochemical classification of the elements proposed by Goldschmidt.1,40 b Cited from the following references: (1) R. Cornelis, X. Fuentes-Arderiu, I. Brunshuus and D. Templeton, Clin. Chim. Acta, 1997, 268, S75–S89. (2) R. Cornelis, E. Sabbioni, and M. T. V. der Venne, Sci. Total Environ., 1994, 158, 191–226. (3) T. Hasegawa, N. Mikuriya, K. Inagaki, E. Fujimori, H. Haraguchi, Y. Nakahara, M. Hattori, T. Kinoshita, and H. Saito, Biomed. Res. Trace Elem., 2000, 11, 455–456. (4) H. Haraguchi et al., “Free Radical and Antioxidant Protocols”, ed. by D. Armstrong, Humana Press, Totowa, 1998, pp. 389–411. (5) K. Inagaki and H. Haraguchi, Analyst, 2000, 125, 191–196. (6) J. Riondato, F. Vanhaecke, L. Moens, and R. Dams, J. Anal. At. Spectrom., 1997, 12, 933–937. (7) W. R. L. Cairns, C. W. Mcleod and B. Hancock, Spectroscopy, 1997, 20, 16. (8) C. S. Phinney, K. E. Murphy, M. J. Welch, P. M. Ellerbe, S. E. Long, K. W. Pratt, S. B. Schiller, L. T. Sniegoski, M. S. Rearick, T. W. Vetter and R. D. Vocke, Fresenius’ J. Anal. Chem., 1998, 361, 71–80. (9) E. Moussa, M. C. R. Demay, F. Veinberg, F. Depasse, R. Gharbi, J. Y. Hautem and P. Aymard, J. Chromatogr. B, 1995, 667, 69–74. c Cited from refs. 37 and 38. | |||
| Si | 0.141 | 2.8 | 0.05 |
| Al | 0.00182 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 03 03 |
60 |
| Fe† | 1.23 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 03 03 |
40![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Ca | 93.13 | 412 | 0.23 |
| Na | 31303 | 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 780 780 |
0.29 |
| Mg | 17.53 | 1280 | 0.014 |
| K | 1513) | 399 | 0.38 |
| Ti | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 006 006![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 5 |
— |
| P† | 1193 | 0.062 | 1920 |
| Mn | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 572 572 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 02 02 |
28.5 |
| F | 0.0191 | 1.3 | 0.015 |
| Ba | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 484 484 |
0.015 | 0.032 |
| Sr | 0.0333 | 7.8 | 0.0085 |
| S†† | 1.116 | 898 | 0.0012 |
| Zr | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 015 015 |
— |
| V | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 0311 0311 |
0.002 | 0.016 |
| Cl | 32008 | 19![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 350 350 |
0.165 |
| Cr | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 0692 0692 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21 21 |
0.33 |
| Rb | 0.173 | 0.12 | 1.42 |
| Ni† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 232 232 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 48 48 |
0.048 |
| Zn†† | 0.6513 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 35 35 |
1860 |
| Ce | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 215 215 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7 7 |
0.0014 |
| Cu†† | 0.753 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15 15 |
5000 |
| Y | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 734 734 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 017 017 |
42.9 |
| La | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 0635 0635 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 005 005![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 6 |
11.2 |
| Nd | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 0345 0345 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 003 003![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 3 3 |
10.3 |
| Co† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 112 112 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2 |
91.6 |
| Sc | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 72 72 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7 7 |
2.43 |
| Li | 0.00164 | 0.18 | 0.0088 |
| N | — | 8.7 | — |
| Nb | — | <0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 005 005 |
— |
| Ga†† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2 |
— |
| Pb†† | 0.00124 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7 7 |
444 |
| B | 0.00212 | 4.5 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 466 466 |
| Pr | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 011 011![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 15 15 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7 7 |
15.85 |
| Th | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 504 504 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 02 02 |
25![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Sm | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 005 005![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 8 5 8 5 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 57 57 |
10.18 |
| Gd | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 007 007![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 25 25 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 9 9 |
8 |
| Dy | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 009 009![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 65 65 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 1 |
8.73 |
| Cs | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 953 953 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 31 31 |
3.06 |
| Yb | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 0135 0135 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2 |
10.8 |
| Hf | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 003 003![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 4 4 |
— |
| Be | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 091 091 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21 21 |
428 |
| Er | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 009 009![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 55 55 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2 |
7.92 |
| Br | 4.442 | 67.0 | 0.0662 |
| Sn†† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 512 512 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 5 |
1020 |
| Ta | — | <0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 5 |
— |
| As†† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 452 452 |
0.0012 | 0.375 |
| U | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 314 314 |
0.0032 | 0.097 |
| Ge†† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 005 005 |
— |
| Mo† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 953 953 |
0.010 | 0.095 |
| W | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 344 344 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 01 01 |
34 |
| Eu | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 825 825 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 17 17 |
4.8 |
| Ho | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 65 65 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 36 36 |
7.2 |
| Tb | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 35 35 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 17 17 |
7.6 |
| I | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 0079 0079 |
0.058 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 12 |
| Tm | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 75 75 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2 |
8.5 |
| Lu | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 55 55 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 23 23 |
10.9 |
| Tl†† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 013 013 |
— |
| Cd†† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 154 154 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 07 07 |
2.1 |
| Sb†† | 0.00233 | 0.0002 | 11.5 |
| Bi†† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 064 064 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 03 03 |
2000 |
| In†† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1 1 |
— |
| Hg†† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 552 552 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 14 14 |
3930 |
| Ag†† | 0.00023 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002 |
10 |
| Se†† | 0.163 | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 16 16 |
1000 |
| Ru† | — | <0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 005 005 |
— |
| Pd† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 06 06 |
— |
| Te†† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 08 08 |
— |
| Pt† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 001 001![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 47 47 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 2 2 |
7.0 |
| Rh† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 08 08 |
— |
| Au† | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 03 03 |
0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 006 006![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 62 62 |
0.0045 |
| Re† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002 |
— |
| Os† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 002 002 |
— |
| Ir† | — | 0.000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 13 13 |
— |
In Table 4, the concentrations of major-to-ultratrace elements in human blood serum are summarized along with those of open sea-water,37,38 where the concentration ratios of the elements in blood serum to those in sea-water are also shown. It is often said that the similarity of the elemental concentrations in human blood serum to those in sea-water is one of the pieces of evidence for the origin of life in the sea.9,39 In order to check their similarities, therefore, the correlation between the concentrations of the elements in human blood serum and sea-water was examined by using the data shown in Table 4. The correlation graph is shown in Fig. 2, where the straight line indicates the equivalent correlation. Therefore, the elements located above the correlation line are at a higher concentration in blood serum than in sea-water, while those below the correlation line are at a higher concentration in sea-water than in blood serum.
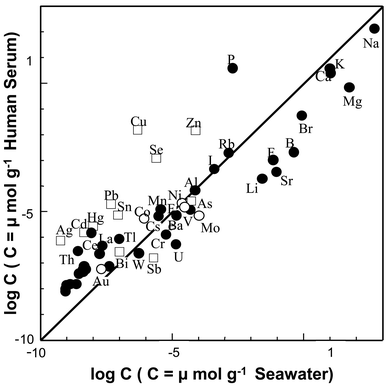 | ||
| Fig. 2 Correlation between the concentrations of the elements in human blood serum and sea-water. ○: Siderophile elements, ●: lithophile elements, □: chalcophile elements. | ||
It should be noted here that the elements in Fig. 2 are shown as three different groups; (i) siderophile elements (○), (ii) lithophile elements (●), and (iii) chalcophile elements (□). The classification of the elements into siderophile, lithophile and chalcophile groups was proposed as the geochemical classification of the elements by Goldshmidt,1,40taking into account the elemental distributions in the whole earth. In general, siderophile and lithophile elements have a greater chemical affinity with oxygen (and carbon) than with sulfur, while chalcophile elements have a greater affinity with sulfur than with oxygen. These chemical properties of the elements can be recognised in the facts that siderophile and lithophile elements are much more abundant in silicate rocks in the lithosphere, and chalcophile elements are abundantly found in sulfide minerals in the earth crust.
It is further noted that the geochemical classification of the elements by Goldsmidt is quite similar to the classification of the elements in the theory of hard and soft acids and bases (HSAB) proposed by Pearson in 1963,41 based on the Lewis acid–base theory. This, in general, says that metal ions in the hard acid group correspond to siderophile and lithophile elements, while those in the soft acid group do so to chalcophile elements. Furthermore, it is known that coordination atoms in hard bases (e.g., O, F, Cl) have the greater affinities with metal ions in the hard acid group, while those in soft bases (S, Br, I) have the greater affinities with metal ions in the soft acid group.
It is seen from Fig. 2 that the elemental distributions in blood serum and sea-water, in general, show the quite good correlation with each other. This fact may support the hypothesis for the origin of life in the ocean. However, the correlation graph shown in Fig. 2 indicates some different or characteristic features in the distributions of the elements in blood serum and sea-water, as summarized below, reflecting the chemical properties of the elements appreciated by the HSAB theory. First, alkali, alkaline earth and halide elements (F, Cl), which are major and minor constituents in both serum and sea-water, are more abundant in sea-water than in blood serum. Second, siderophile and lithophile elements at the trace concentration level are almost equivalently contained in both blood serum and sea-water. Third, chalcophile elements (Cu, Zn, Se, Pb, Sn, Hg, Cd, Ag), which are soft acids, are significantly more abundant in blood serum than in sea-water, which indicates the accumulation of chalcophile elements in human blood serum. Such accumulation of chacophile elements in blood serum can be clearly seen in the concentration ratios shown in Table 4. Among these chalcophile elements, it is seen from Tables 1 and 3 that Cu, Zn, Se, Pb and Sn are presently known to be bio-essential trace elements, which contribute to the bio-active functions in biosynthesis and metabolism in man and mammals. These facts suggest that the primary living organisms on the earth have evolved by utilizing chalcophile elements with sulfur-affinity as essential elements under the anaerobic conditions in the primary atmosphere. It is also noted here that Hg, Cd, Pb and Sn are generally considered to be toxic or hazardous elements, but Pb and Sn are now known to be essential elements for mammals, as is seen in Table 3. However, we should bear in mind that the toxicity or hazardous infection caused by metallic elements inevitably occur whenever any elements are sufficiently accumulated in the cells or organs through overdose or long-term exposure.
4. Distributions of major-to-ultratrace elements in salmon egg cells and their bio-accumulation factors
Many studies on bio-metals have been performed by analyzing various biological samples, such as blood serum, urine, hair and organs.4,5,42 Owing to the extensive development of analytical atomic spectrometry, the multielement determination of major-to-ultratrace elements, even in the biological samples, can be carried out by using ICP-AES and ICP-MS. Then, as mentioned earlier, all-elements analysis of one biological cell is now a challenging subject of analytical biochemistry, which is the final goal of the Extended All Present Theory of the Elements. However, it seems that there has been no study on the multielement determination of major-to-ultratrace elements in one biological cell, which is the basic unit of living organisms such as animals and plants. Thus, the present authors have been conducting research on the determination of all elements in one salmon egg cell. An egg is biologically one cell, in which genes (DNA, RNA), proteins and other biological molecules, as well as various metal ions, must be contained for ontogeny. Thus, many elements contained in an egg cell may be engaged in the various biological functions including syntheses of genes and proteins. Therefore, the distribution measurement of the elements in egg cells is also a research subject of interest in metallomics. Several studies on animal eggs concerned with some bio-essential trace elements, such as Cu, Zn and Fe, have been carried out, for example, on chicken eggs, sea urchins and so forth.43,44The concentrations of major-to-ultratrace elements in whole salmon egg cell are summarized in Table 5,45–47 and were determined by ICP-AES and ICP-MS after acid digestion. The elements in the cell cytoplasm and cell membrane of salmon egg cells were also determined separately in a similar manner to those in the whole egg cell,45 but only the analytical results for whole egg cells are shown in Table 5. The analytical data for 40 elements in whole egg cells, which are shown in Table 5, were obtained using 30 egg cells for analysis.45 When one cell was used for analysis, only 20 elements, which were the first 20 elements, except for Hg, in Table 5, could be determined because of the limited amount of sample.39 However, the concentrations of 20 elements commonly determined in both one egg cell and 30 egg cells were almost consistent with each other (r = 0.98). In addition, when the concentrations of 20 elements in each egg cell for 70 egg cells were independently determined, the relative standard deviations (RSDs) of analytical values for all 20 elements in one egg cell were within 5%.46 Thus, it is quite reasonable to consider that each egg cell contains almost the same amounts of elements when they are collected from the same ovary. The concentration of Hg in cell cytoplasm is additionally shown in Table 5, which was obtained by HPLC-ICP-MS with direct sample injection,48 as will be described later.
| Elementa | Concentrationb/ng g−1 | Bio-concentration factor |
|---|---|---|
| a The elements marked * are bio-essential. b Mean value (n = 3). The concentrations of the elements are expressed on a wet-weight basis, and were determined using thirty egg cells for one analysis. c The value for cell cytoplasm, which was determined by the flow injection method after 5-times dilution with 0.1 mM Tris-HCl buffer solution (pH 7.4). | ||
| P* | 3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 840 840![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
62![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| K* | 2![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 440 440![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
6.1 |
| Mg* | 857![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
0.67 |
| Ca* | 502![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
1.22 |
| Na* | 435![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
0.040 |
| Zn* | 16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700 700 |
47![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700 700 |
| Fe* | 15![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 800 800 |
527![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Cu* | 8050 | 53![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700 700 |
| Sr* | 6710 | 0.86 |
| Mn* | 1400 | 70![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Rb | 741 | 6.2 |
| Ba | 220 | 14.7 |
| Ag | 41.0 | 20![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 500 500 |
| Ni* | 37.9 | 79 |
| Co* | 33.0 | 27![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 500 500 |
| Hg | (12.0)c | 120![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Mo* | 14.0 | 1400 |
| Cs | 12.3 | 39![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700 700 |
| Cd | 3.45 | 49![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 300 300 |
| Sn | 2.93 | 5![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 860 860![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| U | 2.39 | 747 |
| Tl | 0.351 | 27![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Y | 0.237 | 13![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 900 900 |
| Nd | 0.181 | 54![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 800 800 |
| La | 0.177 | 31![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 600 600 |
| Ce | 0.114 | 163![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Sm | 0.091 | 160![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Gd | 0.063 | 70![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Eu | 0.056 | 329![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| W | 0.050 | 5000 |
| Dy | 0.046 | 41![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 800 800 |
| Pr | 0.036 | 51![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 400 400 |
| Th | 0.026 | 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 300 300![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Yb | 0.018 | 15![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 000 |
| Er | 0.015 | 12![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 500 500 |
| Ho | 0.012 | 33![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 400 400 |
| Tb | 0.0083 | 48![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 800 800 |
| Tm | 0.0061 | 30![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 500 500 |
| Lu | 0.0043 | 18![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700 700 |
It is seen in Table 5 that the concentration of P was the maximum (3![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 840
840![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 ng g−1) in whole egg cell, while that of Lu was the minimum (0.0043 ng g−1). The concentrations of Zn, Cu and Fe in whole egg cell were 16
000 ng g−1) in whole egg cell, while that of Lu was the minimum (0.0043 ng g−1). The concentrations of Zn, Cu and Fe in whole egg cell were 16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700, 8050 and 15
700, 8050 and 15![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 800 ng g−1, respectively.
800 ng g−1, respectively.
The bio-concentration factor (or bio-accumulation factor) of the element in marine organisms, which is estimated as the concentration ratio of the elements in a marine organism to that in sea-water, is often used as the indicator of accumulation of pollution by the element in the marine organism from sea-water. Thus, the bio-concentration factors of the elements in salmon egg cells were estimated using the data for sea-water, which are shown in Table 4. The concentrations of Zn, Cu and Fe in salmon egg cells were 16![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 700, 8050 and 15
700, 8050 and 15![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 800 ng g−1, respectively, which correspond to the bio-concentration factors of ca. 48
800 ng g−1, respectively, which correspond to the bio-concentration factors of ca. 48![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000, 54
000, 54![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000 and 530
000 and 530![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000, respectively. The bio-concentration factors for P, Mn, Co, Sn and Ag were also larger than 20
000, respectively. The bio-concentration factors for P, Mn, Co, Sn and Ag were also larger than 20![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000. These results suggest that salmon egg cells selectively accumulate the large amounts of bio-essential elements for ontogeny, although the bio-essentiality of Ag is unknown so far. In addition, it can be noted here that the concentration and bio-concentration factors of Hg in salmon egg cells were 12 ng g−1 and 120
000. These results suggest that salmon egg cells selectively accumulate the large amounts of bio-essential elements for ontogeny, although the bio-essentiality of Ag is unknown so far. In addition, it can be noted here that the concentration and bio-concentration factors of Hg in salmon egg cells were 12 ng g−1 and 120![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000, respectively. It is also seen from Table 4 that the bio-concentration factors of rare earth elements were also very large (>100
000, respectively. It is also seen from Table 4 that the bio-concentration factors of rare earth elements were also very large (>100![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000), although their biological functions have been unknown up to now. The largest bio-concentration factor was obtained for Th (1
000), although their biological functions have been unknown up to now. The largest bio-concentration factor was obtained for Th (1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 300
300![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000). On the other hand, Mo, U and W, which exist in the oxoanion or oxocation forms in sea-water, gave bio-concentration factors smaller than 10
000). On the other hand, Mo, U and W, which exist in the oxoanion or oxocation forms in sea-water, gave bio-concentration factors smaller than 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000.
000.
Some typical metalloenzymes (and metalloproteins) were shown in Table 1 together with their molecular weights and functions. It can be seen from Table 1 that DNA polymerase and RNA polymerase are zinc-enzymes, which regulate syntheses of genes. Therefore, it is considered that Zn accumulated in salmon egg at a substantially high concentration level may play a potential role in DNA/RNA syntheses as well as in other biological functions, such as biological energy production (ATPase) and cell division regulation. Phosphorus is a component of DNA, RNA, ATP or phospholipids, although about 98% of P in salmon egg was inorganic phosphate ion (PO43−).47 It is known that magnesium forms some complexes with nucleic acids for stabilization of their three-dimensional structures, and also that calcium easily combines with proteins at the carbonyl terminals. Iron is the component of blood. Thus, the markedly high concentrations of Zn, P, Mg, Ca, Fe, and Cu in salmon egg suggests their significant roles in inducing various physiological functions.
5. Speciation of the elements in biological samples by surfactant-mediated HPLC-ICP-MS
In the speciation study on trace elements in biological and other samples, various separation techniques, such as LC, GC and capillary electrophoresis, have been used in combination with the element-selective detection methods1,11–13,30,49,50 as mentioned earlier. In the case of LC, various kinds of separation columns are usually employed, depending on the chemical properties of analytes. Here, chemical speciation of trace metals binding with proteins and DNA/RNA using a surfactant-mediated separation column will be described. As the surfactant-mediated separation column, an ODS (octadecylsilica) column coated with CHAPS ([(3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), a zwitterionic bile acid derivative, was used, which was developed in the present author's laboratory as a versatile separation column. The CHAPS-coated ODS column was prepared by a dynamic coating method, where the ODS column (L-column; 4.6 id ×250 mm long) was purchased from Chemicals Evaluation and Research Institute, Tokyo, Japan. It is noted here that the CHAPS-coated ODS column provides excellent separation characteristics;51–55 it allows us to separate large and small molecules simultaneously and rapidly with direct injection of biological fluid samples, such as blood serum and urine, without any pretreatment. Such excellent separation characteristics are based on the mixed separation modes, in which large molecules are eluted by a size-exclusion mode,53–58 while small molecules sre separated by electrostatic and/or hydrophobic interactions.51–53 As a result, large molecular species with molecular weights larger than 10 kDa are eluted within ca. 2.5 min with no retention, while small molecular species with molecular weights smaller than 10 kDa are eluted after ca. 2.5 min, but within 10 min. So far, the present surfactant-mediated HPLC coupled with ICP-MS has been applied to the speciation of trace elements55 and cisplatin56,57 in human blood serum as well as to the speciation of some small molecules or inorganic ions in salmon egg cells.475.1. Speciation of cisplatin in human blood serum
Cisplatin, (cis-dichlorodiammineplatinum(II)), is clinically used as an anti-cancer drug due to its antitumor effects.58,59 However, cisplatin causes kidney infection, which is serious side-effect so often proving to be fatal. Thus, the antitumor effects of cisplatin as well as reduction of its side-effect have been extensively investigated to develop more efficient chemotherapic treatment methods for cancer.59–62. Usually, cisplatin is dosed to the cancer patients by vein injection, and then it is transported with blood serum to the organs in the body. Therefore, many speciation studies on cisplatin and other platinum compounds in relation to the metabolites of cisplatin, as well as its dissolving states in blood, have been conducted.63–65 As a result, cisplatin can form various types of complexes with amino acids65as well as proteins.66 Even in such present situations described above, the convenient methods for clinical analysis are still required in medical diagnosis and chemotherapy. Thus, chemical speciation of cisplatin in human blood serum has been carried out using the CHAPS-coated ODS column in the following experiment.In Fig. 3, the time-dependent chromatograms of cisplatin added in human blood serum are shown, which was obtained by the surfactant-mediated HPLC-ICP-MS system with 195Pt detection. Since proteins in blood serum were not adsorbed or retained on the CHAPS-coated ODS column, blood serum could be directly injected into the column without any sample pretreatment. It is clearly shown in Fig. 3 that free cisplatin and protein-binding cisplatin were observed at retention times of 5.7 min and 2.5 min, respectively. It is noted here that ca. 40% of cisplatin binds with proteins (mostly serum albumin) in blood serum within 1 h, and it binds almost completely with proteins after 25 h. In addition, some small peaks are observed near the retention time of ca. 4.0 min in the chromatograms in Fig. 3, which were identified as cysteine complexes of cisplatin and its hydrolysis-type complexes.56
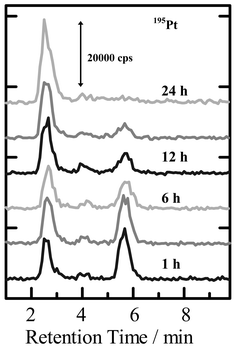 | ||
| Fig. 3 Time-dependent chromatograms of cisplatin added in human blood serum with 191Pt detection by ICP-MS. Sample: human blood serum with 600 ng g−1 of cisplatin added; column: CHAPS-coated ODS column; mobile phase: 0.2 mM Tris-HCl buffer with 0.2 mM CHAPS added (pH 7.4); flow rate: 0.7 ml min−1; injection volume: 20 µl. The numbers (1 h, 6 h, 12 h, 24 h) in the figure indicate the times after addition of cisplatin in blood serum. | ||
5.2. Bindings of cisplatin with DNA and RNA
It has been generally considered that the antitumor effect of cisplatin is caused by its binding with DNA in cancer cells. Thus, the binding of cisplatin with DNA has been investigated by NMR and other methods.59,67–70 In the present experiment, the surfactant-mediated HPLC-ICP-MS system using the CHAPS-coated ODS column was applied to examine the bindings of cisplatin with DNA and RNA. The chromatograms of cisplatin added to 0.05 % DNA and RNA aqueous solutions are shown in Fig. 4, where DNA and RNA were detected by 31P and cisplatin was detected by 195Pt. In 31P-detected chromatograms for DNA and RNA, the large peaks derived from P in nucleic acids were observed near 3.0 min. On the other hand, the peaks for Pt in free cisplatin were mainly observed near 6.5 min, 1 h after addition of cisplatin, in 195Pt-detected chromatograms of both of DNA and RNA solutions. It is clearly seen in the 195Pt-detected chromatograms measured after 25 h that the new peaks were observed near 3.0 min, which was the same retention time as found in the 31P-detected chromatograms of DNA and RNA. Thus, these results indicate that cisplatin certainly binds not only with DNA, but also with RNA. It is also noted here that cisplatin binds relatively more with DNA than with RNA, as is seen in Fig. 4. In addition, the small peaks are observed between 4–6 min in the 195Pt-detected chromatograms, which were ascribed to hydrolysis-type complexes such as Pt(NH3)2(H2O)(OH) and Pt(NH3)2(OH)2.56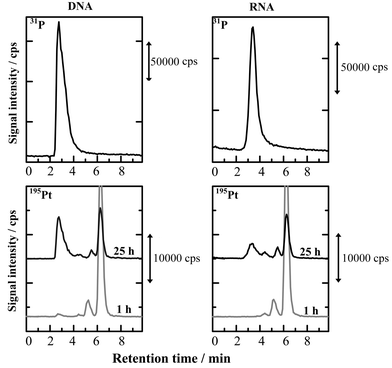 | ||
| Fig. 4 Chromatograms of cisplatin in DNA and RNA aqueous solutions with 31P- and 195Pt-detections by ICP-MS. Samples: 0.05% DNA and 0.05% RNA aqueous solutions added with 600 ng ml−1 of cisplatin. Other experimental conditions were the same as in Fig. 5. | ||
5.3. Speciation of mercury in cytoplasm of salmon egg cell
The UV-absorption and Hg-detected chromatograms for cytoplasm of salmon egg cell are shown in Fig. 5, where the CHAPS-coated ODS column was also used for separation. In the experiment shown in Fig. 5, cytoplasm, extracted from the cells manually, was diluted 5-times with 0.1 mM Tris-HCl buffer solution (pH 7.4), and the diluted solution was directly injected onto the separation column. It is seen from the UV-absorption chromatogram in Fig. 5 that large absorption peak due to proteins are observed near 3 min, and several small peaks due to some other small molecules were after 3 min. In the chromatograms for Hg detected by ICP-MS, the Hg peaks are mainly observed at the same retention time as those of proteins detected by UV-absorption at 254 nm. In addition, the relative peak ratios between 199Hg, 200Hg, 201Hg and 202Hg in the Hg-detected chromatograms were almost consistent with those estimated from their natural abundances. From these experimental results, it was concluded that Hg-binding proteins existed in the cytoplasm of salmon egg cell, although no attempt to identify those proteins was made in the present experiment. The total concentration of Hg in cytoplasm was 12 ng g−1, which was determined by a flow injection method in a separate experiment.The chromatograms for the cytoplasm of salmon egg cell are shown in Fig. 6, which were also observed by the surfactant-mediated HPLC-ICP-MS system with 202Hg and S(32S16O) detection together with the UV-absorption. In the experiment shown in Fig. 6, cytoplasm of salmon egg cell diluted 5-times with 0.01 mM Tris-HCl buffer solution (pH 7.4) was used for analysis. It should be noted here that the large peak was deleted in the UV-absorption chromatogram and only the peaks corresponding to small molecules were retained. This experimental result indicates that the dilution of cytoplasm with 0.01 mM Tris-HCl buffer solution caused deproteinization, so that only a Hg peak bound with small molecules was detected in the 195Pt- and S-detected chromatograms in Fig. 6. The relatively large peak detected near 5.5 min in the S-detected chromatogram was due to molecular ion ascribed to 40Ca16O.
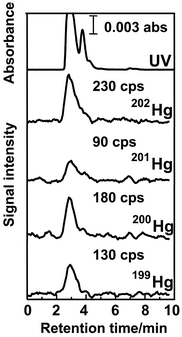 | ||
| Fig. 5 Chromatograms for cytoplasm of salmon egg cell with detection by UV absorption and ICP-MS. Sample: cytoplasm of salmon egg cell diluted 50 times with 0.1 mM Tris-HCl buffer (pH 7.4). Column, CHAPS-coated ODS; mobile phase, 0.2 mM Tris-HCl buffer with 0.2 mM CHAPS; flow rate, 0.7 ml min−1; injection volume, 20 µl; absorption wavelength, 254 nm. | ||
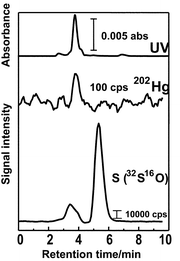 | ||
| Fig. 6 Chromatograms of cytoplasm of salmon egg cell, with detection of UV absorption, 208Hg and S(32S16O). Sample: cytoplasm of salmon egg cell diluted 5-times with 0.01 mM Tris-HCl buffer. Other experimental conditions were the same as in Fig. 5. | ||
It is seen in Fig. 6 that Hg was detected at 3.8 min, which was consistent with the peaks observed in the chromatograms detected by UV absorption and S(32S16O). In order to identify the peak at 3.8 min, the spiked experiment with addition of cysteine in cytoplasm diluted 5-times with 0.01 mM Tris-HCl buffer was carried out by S(32S16O) detection. From this spiked experiment, the peak at 3.8 min was identified as cysteine. Then, it was concluded that the peak at 3.8 min in the 202Hg-detected chromatogram in Fig. 6 was ascribed to the Hg–cysteine complex, which was observed as the small shoulder peak in Fig. 5. The concentration of Hg in cysteine complexes was also determined by the flow injection method, and it was 3.7 ng g−1.
From the experimental results described above, it was found that Hg existed as protein- and cysteine-bound complexes in the cytoplasm of a salmon egg cell. As mentioned before, the total concentration of Hg in cytoplasm was 12 ng g−1, and then it could be estimated that the concentration of Hg in protein-complexes was 8.7 ng g−1. The research on the identification of proteins binding with Hg in salmon egg cell is now going on in our laboratory.
Although the results are not shown here, for example, it was also found that the oxidation states of As were mainly +5 (mainly arsenide) and +3 (mainly arsenobetaine) in cell membrane and cytoplasm, respectively, according to our recent work on the chemical speciation of As using ion-pair formation chromatography with As-detection by ICP-MS.71
6. Conclusion
Metallomics is proposed as the interdisciplinary research field for the promotion of biometal science. Metal ions substantially play biologically important roles in maintaining the physiological functions in our life systems, keeping us healthy under the homeostasis conditions. However, the science of bio-metals has not received great attention as a systematic scientific field because the scientific researches on biometals have been carried out independently or separately in many scientific fields. In the present paper, several research topics, such as the distributions of the elements in man, human blood serum and open sea-water, and the challenge of all-elements analysis of one biological cell, were introduced, which could be obtained owing to recent development of analytical atomic spectrometry. In addition, some works on the chemical speciation of cisplatin in human blood serum and DNA/RNA solutions and mercury in the cytoplasm of salmon egg cells were described, where the surfactant-mediated HPLC column (CHAPS-coated ODS column) was used to observe large and small molecules simultaneously. Such examples in analytical biochemistry will certainly encourage us to explore the new scientific fields. Metallomics is one of these new scientific fields, and it is desirable to establish metallomics as the systematic academic field to elucidate the intrinsic mechanisms of the biological functions as well as chemical evolution of the biological systems.Finally, it should be stated that metallomics is the scientific field of post-genomes and/or post-proteomes, but it should be stressed here that metallomics is the scientific field to be developed in co-operation with genomics, proteomics and metabollomics.
Acknowledgements
The author expresses sincere thanks to Dr. Akihide Itoh, Mr. Takuya Hasegawa, Mr. Kouhei Takatani, Ms. Hitomi Nagata and Mr. Motoki Asano for providing the experimental data and collection of geochemical data. I also express deep appreciation to Mss Yoko Nagata and Haruko Ogiso for their help in preparation of the manuscript. The present research was supported partly by the Scientific Grant-in-Aid (No. 13450346) and also partly by the Scientific Grant for the 21st Century Programs “Future-oriented Isotope Science” and “Nature-guided Materials Processing” from the Ministry of Education, Culture, Sports, Science and Technology, Japan.References
- H. Haraguchi, Bull. Chem. Soc. Jpn., 1999, 72, 1163–1186 CrossRef CAS.
- C. Vandecasteele and C. B. Block, Modern Methods for Trace Element Determination, John Wiley, Chichester, UK, 1993 Search PubMed.
- H. Sakurai and H. Tanaka, Bio-trace Elements, Hirokawa-shoten, Tokyo, 1994 (in Japanese) Search PubMed.
- J. Versieck and R. Cornelis, Trace Elements in Human Plasma and Serum, CRC Press, Boca Raton, FL, 1989 Search PubMed.
- A. Aitio, A. Aro, J. Jarvisalo and H. Vairio, Trace Elements in Health and Disease, The Royal Society of Chemistry, Cambridge, UK, 1991 Search PubMed.
- Trace Elements in Clinical Medicine, ed. H. Tomita, Springer-Verlag, Tokyo, 1990 Search PubMed.
- S. J. Lippard and J. M. Berg, Principles of Bioinorganic Chemistry, University Science Book, Princeton, NJ, USA, 1994 Search PubMed.
- Inorganic Biochemistry, ed. G. L. Eichhorn, Elsevier Scientific, Amsterdam, 1973, vol. 1, 2 Search PubMed.
- A. M. Faibane and D. R. Williams, The Principle of Bio-inorganic Chemistry, Chemical Society Monographs for Teachers, No. 31, The Chemical Society, London, UK, 1981 Search PubMed.
- I. S. Knull, Trace Metal Analysis and Speciation, Elsevier, Amsterdam, 1991 Search PubMed.
- J. Szpunar and R. Lobinski, Pure Appl. Chem., 1999, 71, 899–918 CAS.
- J. Szpunar, Analyst, 2000, 125, 963–988 RSC.
- J. Szpunar and R. Lobinski, Anal. Bioanal. Chem., 2002, 373, 404–411 CrossRef CAS.
- M. Montes-Bayon, K. DeNicola and J. A. Caruso, J. Chromatogr. A, 2003, 1000, 457–476 CrossRef CAS.
- J. Szpunar, R. Lobinski and A. Prange, Appl. Spectrosc., 2003, 57, 102A–112A CrossRef CAS.
- J. A. Caruso and M. Montes-Bayon, Ecotox. Environ. Safe., 2003, 56, 148–163 CrossRef CAS.
- M. Montes-Bayon, Anal. Bioanal. Chem., 2003, 376, 287–288 CAS.
- K. E. Jarvis, A. L. Gray and R. S. Houk, Handbook of Inductively Coupled Plasma Mass Spectrometry, Blackie, Glasgow, UK, 1992 Search PubMed.
- Inductively Coupled Plasma Mass Spectrometry, ed. A. Montaser, VCH Publishers, New York, 1998 Search PubMed.
- H. Haraguchi and H. Sawatari, Clin. Neurosci., 1994, 12, 146–148 Search PubMed (in Japanese).
- H. Haraguchi, K. Inagaki, A. Hokura and H. Matsuura, Trace Nutritions Res., 1998, 15, 11–22 Search PubMed (in Japanese Biryo Eiyo-so).
- H. Haraguchi and T. Hasegawa, Biomed. Res. Trace Elem., 2002, 13, 31–45 Search PubMed.
- I. Noddack, Angew. Chem., 1936, 47, 835 Search PubMed.
- P. K. Kuroda, Origin of the Chemical Elements and Oklo Phenomenon, Springer-Verlag, Berlin, 1982 Search PubMed.
- H. Haraguchi and H. Matsuura, Proceedings of International Symposium on Bio-Trace Elements 2002 (BITRE 2002), eds. S. Enomoto and Y. Seko, The Institute of Physical and Chemical Research (RIKEN), Wako, 2003, pp. 3-8 Search PubMed.
- R. J. P. Williams, Coord. Chem. Rev., 2001, 216, 583–595 CrossRef.
- Science, 2003, 300, 925–947 Search PubMed.
- H. Haraguchi and H. Sawatari, Clin. Neurosci., 1994, 12, 925 Search PubMed (in Japanese).
- J. C. Lindon, E. Holmes and J. K. Nicholson, Anal. Chem., 2003, 75, 385A–391A.
- H. Tao, Bunseki Kagaku, 1997, 46, 239–263 CAS.
- J. Szpunar, S. McSheehy, K. Poles, V. Vacchina, S. Mounicou, I. Rodriguez and R. Lobinski, Spectrochim. Acta, 2000, 55, 779–793.
- J. R. Ecinar, L. Querdane, W. Buchmann, J. Tortajada, R. Lobinski and J. Spunar, Anal. Chem., 2003, 75, 3765–3774 CrossRef.
- S. McSheehy, M. Marcinek, H. Chassaigne and J. Szpunar, Anal. Chim. Acta, 2000, 410, 71–84 CrossRef CAS.
- K. Palec-Pawlak, D. Schaumloffel, J. Szpunar, A. Prange and R. Lobinski, J. Anal. At. Spectrom., 2002, 17, 880–886 RSC.
- E. Rosenberg, J. Chromatogr. A., 2003, 1000, 841–889 CrossRef CAS.
- Bio-trace Elements, eds. H. Sakusai and H. Tanaka, Nankodo, Tokyo, 1996 Search PubMed.
- Y. Nozaki, EOS Trans., 1997, 78, 221 Search PubMed.
- Y. Nozaki, Bull. Soc. Sea Water Sci. Jpn., 1997, 51, 302–338 Search PubMed.
- M. Calvin, Chemical Evolution–Molecular Evolution towards the Origin of Living Systems on the Earth and Elsewhere, Oxford University Press, Oxford, UK, 1969 Search PubMed.
- V. M. Goldschmidt, Geochemistry, ed. A. Muir, Clarendon Press, Oxford, 1954 Search PubMed.
- R. G. Pearson, J. Am. Chem. Soc, 1963, 85, 3533 CrossRef CAS.
- H. Haraguchi, E. Fujimori and K. Inagaki, Free Radical and Antioxidant Protocols, ed. D. Armstrong, Humana Press, Toronto, 1998, pp. 389–411 Search PubMed.
- J. C. A. Craik and S. M. Harvey, Aquaculture, 1988, 73, 309 CrossRef.
- R. Scudiero, C. Capasso, P. P. Deprisco, A. Capasso, S. Filosa and E. Parisi, Cell Biol. Int., 1994, 18, 47 CrossRef CAS.
- H. Matsuura and H. Haraguchi, Anal. Sci., 2001, 17(Supplement), 1975–1978.
- H. Matsuura and H. Haraguchi, Biomed. Res. Trace Elem., 2002, 13, 230–231 Search PubMed.
- H. Matsuura, T. Hasegawa, H. Nagata, K. Takatani, M. Asano, A. Itoh and H. Haraguchi, Anal. Sci., 2003, 19, 117–121 CAS.
- M. Asano, H. Matsuura and H. Haraguchi, unpublished data.
- E. G. Yanes and N. J. Miller-Ihli, J. Anal. At. Spectrom., 1999, 14, 1697–1702 RSC.
- Y. M. Liu and J. K. Cheng, Electrophoresis, 2003, 24, 1993–2012 CrossRef CAS.
- W. Hu, T. Takeuchi and H. Haraguchi, Anal. Chem., 1993, 65, 2204–2208 CAS.
- W. Hu, H. Tao and H. Haraguchi, Anal. Chem., 1994, 66, 2514–2520 CrossRef CAS.
- T. Umemura, R. Kitaguchi and H. Haraguchi, Anal. Chem., 1998, 70, 936–942 CrossRef CAS.
- T. Umemura, R. Kitaguchi, K. Inagaki and H. Haraguchi, Analyst, 1998, 123, 1767–1770 RSC.
- K. Inagaki, T. Umemura, H. Matsuura and H. Haraguchi, Anal. Sci., 2000, 16, 787–788 CAS.
- H. Haraguchi, T. Ohshima, H. Matsuura and T. Hasegawa, Biomed. Res. Trace Elements, 2001, 12, 128–140 Search PubMed.
- H. Haraguchi, T. Ohshima, H. Matsuura and T. Hasegawa, Anal. Sci., 2001, 17(Supplement), 137–140.
- B. Rosenberg, L. Van Camp and L. Krigas, Nature, 1965, 205, 698–699 CAS.
- E. R. Jamieson and S. J. Lippard, Chem. Rev., 1999, 99, 2467–2498 CrossRef CAS.
- T. Oe, Y. Tian, P. J. O'Dwyer, D. W. Roberts, C. J. Bailey and I. A. Blair, J. Chromatogr. B, 2003, 792, 217–227 CrossRef CAS.
- C. J. Smith, I. D. Wilson and F. Abou-Shakra, Anal. Chem., 2003, 75, 1463–1469 CrossRef CAS.
- I. A. Blair and A. Tilve, Curr. Drug Metab., 2002, 3, 463–480 Search PubMed.
- W. R. L. Cairns, L. Ebdon and S. J. Hill, Fresenius’ J. Anal. Chem., 1996, 355, 202–208 CAS.
- R. R. Barefoot, J. Chromatogr. B, 2001, 751, 205–211 CrossRef CAS.
- M. El-Khateeb, T. G. Appleton, L. R. Gahan, B. G. Charles, S. J. Berners-Price and A. M. Bolton, J. Inorg. Biochem., 1999, 77, 13–21 CrossRef CAS.
- T. P. Shulman, Y. Najajreh and D. Gibson, J. Inorg. Biochem., 2002, 91, 306–311 CrossRef CAS.
- A. M. J. Fichtinger-Schepman, J. L. van der Veer, J. H. J. den Hartog, P. H. M. Lohman and J. Reedijik, Biochemistry, 1985, 24, 707–713 CrossRef CAS.
- J. F. Hartwig and S. J. Lippard, J. Am. Chem. Soc., 1992, 114, 5646–5654 CrossRef CAS.
- P. M. Pil and S. J. Lippard, Science, 1992, 256, 234–237 CAS.
- A. Gelaso and S. J. Lippard, Biochemistry, 1998, 37, 9230–9239 CrossRef CAS.
- H. Matsuura and H. Haraguchi, Biomed. Res. Trace Elem. Search PubMed , in the press.
| This journal is © The Royal Society of Chemistry 2004 |
