David E. Bergbreiter and Steven Furyk
Green Chem., 2004,6, 280-285
DOI:
10.1039/B316342C,
Paper
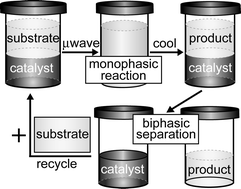
Palladium catalyzed Heck couplings utilizing an air-stable, water-soluble oligo(ethylene glycol)-bound SCS palladacycle