Microwave assisted synthesis – a critical technology overview
M.
Nüchter
a,
B.
Ondruschka
a,
W.
Bonrath
b and
A.
Gum
b
aInstitute of Technical Chemistry and Environmental Chemistry, Friedrich-Schiller-University Jena, Lessingstr. 12, D-07743 Jena, Germany
bDMS Nutritional Products, Research & Development, P.O. Box 3255, CH-4002 Basel, Switzerland
First published on 26th February 2004
Abstract
Since 1986, when Gedye and Giguere published their first articles in Tetrahedron Letters on microwave assisted syntheses in household microwave ovens, there has been a steadily growing interest in this research field. Since the use of microwaves comprises more than the simple application of a goal-oriented, innovative tool, it is crucial to be aware of the fundamentals of chemistry in the microwave field before investigating challenging reaction mechanisms. Therefore, the following overview focuses mostly on reaction engineering in the microwave field. Parallel reactions and scale-up are also discussed. The last section of this review is dedicated to the development of experimentally sound protocols on microwave-assisted syntheses and separations, which are illustrated through experiments.
1 Introduction
The use of microwaves as an energy source for chemical reactions and processes has been extensively investigated during recent years,1–4 but has also led to many controversial discussions.5–7 A great number of scientific publications cover this subject. An extensive but incomplete overview was previously published.2g The critical net result of recent publications leads to several common points:– There is hardly any reaction type or name reaction that has not yet been tested in the microwave field.
– Independent of the impact factor of the scientific journal or its referee system, the experimental details and the microwave systems used are usually insufficiently described. Often, other research groups have great trouble reproducing the attractive reaction parameters such as conversion, selectivity and yield.
– No reaction exists that only proceeds in the microwave field. There are always similar reactions under classical conditions, i.e. thermal heating.
Recently, articles such as reference8a in the “highlights” section of a respected journal (cf. the correspondence letter8b) and 9–11 were published, which led to discussions on these and points of view presented in these articles. This article reviews the essential aspects of the use of non-classical energy input. The presented arguments will be supported through experimental evidence.
Fig. 1 summarizes the development of publications on microwave-assisted reactions since 1985.12 The first chemical reactions relating to organic synthesis date back to 1986.13,14
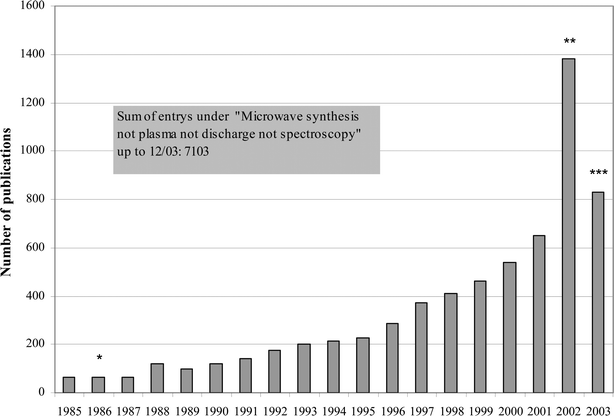 | ||
| Fig. 1 Number of publications identified by keyword “microwave synthesis …”12 plotted against publication year * Includes only two publications about organic synthesis. ** Includes 483 chinese patents concerning microwave assisted extraction of medicine plants. *** Search results up to December 2003. | ||
Such a figure is often found in review articles, cf.2g. However, the number of papers published on a certain subject does not address the quality of those investigations. A quality criterion for the level of understanding of the subject has not yet been established.
The use of microwaves for the activation of chemical reactions in a broader sense has been known for a long time. A great number of applications, such as sintering, drying, melting and defrosting have been reported. Rapid developments in those fields still prevail today. In contrast to many other subjects investigated during recent years, one cannot talk about a “temporary fashion” in this case. This is due to the presented, partly surprising, results and the innovations that require the use of microwave-activation.
As opposed to conventional thermal heating, the use of microwave radiation for the “activation” of chemical reactions requires a certain theoretical preparation. More than ever, the synthetic chemist experiences measurement problems and physical problems when using microwaves. This makes the connection of classical chemical synthesis to technical engineering sciences unavoidable. The fact that more measurement technology will be required is contradictory to the current trends in simplification and miniaturisation (making certain operations, e.g. stirring, superfluous) observed in organic synthesis. Many publications in the literature do not provide essential reaction parameters. While the analytical equipment (NMR, GC) used is often reported in detail, the description of the microwave devices employed are rarely documented (e.g.15–17). Often, the type of the microwave device, typically a domestic microwave oven, is not even mentioned. The power used is described in terms of “full power” or only the preset power step is given. Thus, it is impossible to draw conclusions about the achieved temperatures. Furthermore, the reactions performed are often only compared to literature data from reactions that were performed under completely different conditions. For scale-up of first laboratory results (in mmol scale), it is necessary to describe the experimental details, e.g. apparatus, reaction protocol, very carefully. This is a general rule for all process development, and not only connected to chemistry under microwave conditions. A disadvantage of microwave technology is perhaps the high investment costs.
Interestingly, microwaves, energy sources that have been previously used for decades for rather “trivial” applications such as cooking, heating of food, drying etc., have also been used for research purposes.18 The microwave devices utilized up to today have high security standards with respect to electromagnetic radiation, but are of limited use for chemical reactions. The control and setting of reaction parameters is limited to the energy input and the irradiation time. Pressure and temperature measurements are extremely problematic. This makes a comparison with classical reaction conditions difficult and leads to speculations about non-thermal (or microwave) effects.19–24 Since the reaction control is only performed by energy input, temperature limitation is not possible by switching the power off.
In recent years, the technical developments, e.g. possibilities for temperature and pressure measurement, continuous processes, unpulsed energy entry, microwave leak sensors, in specialized systems that are adapted to chemical synthesis have extended the possibilities of microwave-assisted reaction engineering.
With these new advantages, there is a growing interest in scaling-up this method. When discussing the advantages of the microwave-assisted power input into chemical reactions and processes, one must always consider that the energy of microwave quanta is far too small to directly initiate a chemical reaction. Based on data for the bond energy it must be pointed out that microwave irradiation does not give a high enough energy to break typical chemical bonds commonly found in organic synthesis (C–C, C–O, or C–H, Table 1).
| Energy/eV b | Energy/kJ mol−1 | ||
|---|---|---|---|
| a For more examples of strengths of chemical bonds see ref. 73. b 1 eV = 1.602177 × 10−19 J. c See ref. 74. d See ref. 75. | |||
| 1 | CC single bond | 3.61c | 347 |
| 2 | CC double bond | 6.35c | 613 |
| 3 | CO single bond | 3.74c | 361 |
| 4 | CO double bond | 7.71c | 744 |
| 5 | CH bond | 4.28c | 413 |
| 6 | OH bond | 4.80c | 463 |
| 7 | hydrogen bond | 0.04–0.44d | 4–42 |
| 8 | MW 0.3 GHz | 1.2 × 10−6 | 0.00011 |
| 9 | MW 2.45 GHz | 1.0 × 10−5 | 0.00096 ≈ 1 J mol−1 |
| 10 | MW 30 GHz | 1.2 × 10−3 | 0.11 |
2 Principles of microwave irradiation
2.1 Physical principles – energy conversion – penetration depth
The physical principles of microwaves are based on relatively simple laws and are described briefly in the following paragraphs.The wavelength λ0 of a microwave (in this case 12.24 cm) is related to the frequency (2.45 GHz) viaeqn. (1). The frequency indicates the number of oscillations of the electric or magnetic field in one second, cf.1f
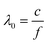 | (1) |
The mechanism by which matter absorbs microwave energy is called dielectric heating.3b In this context, an important property is the mobility of the dipoles and the ability to orient them according to the direction of the electric field. The orientation of the dipoles changes with the magnitude and the direction of the electric field. Molecules that have a permanent dipole moment are able to align themselves through rotation completely or at least partly with the direction of the field. Molecules can rotate in time with field frequencies of 106 Hz in gases or liquids.2c However, they cannot follow the inversion of the electric field at an indefinite time. Phase shifts and dielectric losses are the results. In this case, besides the dielectric coefficient (permittivity), the size (mass) of the excited molecules is also relevant. Field energy is transferred to the medium and electrical energy is converted into kinetic or thermal energy. Molecular friction is often cited as a model for this behaviour. For numerous polar substances, dielectric losses are observed in the microwave range.2c
A simplified illustration of the heating mechanism of polar solvents by microwave radiation is provided in Fig. 2 for the example of a water molecule.
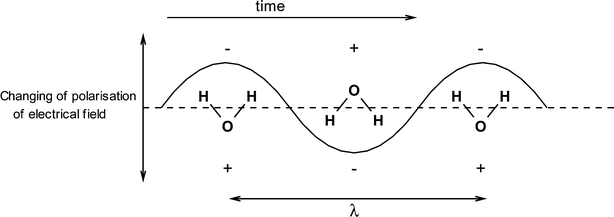 | ||
| Fig. 2 Water in an alternating electrical field. | ||
The fast changing electric field of the microwave radiation leads to a rotation of the water molecules. Due to this process, “internal friction” takes place in the polar medium, which leads to a direct and almost even heating of the reaction mixture. Because the change in the polarity of the electric field is faster than the rotation of the water molecules around its dipole centre, a phase shift results and energy is absorbed from the electric field.
Reflections and refractions on local boundaries yield “hot spots” and may result in a “super-heating” effect, which has been controversially discussed in the literature.25,26 This effect can be described best as local overheating and is comparable to the delayed boiling of overheated liquids under conventional conditions. This effect is characteristically found only in unstirred solutions.
The coupling of microwave energy in the medium depends on the dielectric properties of the substance to be heated, i.e. it depends on the quantity of microwave radiation that fails to penetrate the substance.2c A measure of this behaviour is the dielectric coefficient εr that is characteristic for each substance and its state. εr is related to the capacity C, i.e. the ability to save electric energy, viaeqn. (2) (capacitor model):
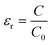 | (2) |
For the electromagnetic field, εr is extended by the imaginary part iεr″ according to eqn. (3), where i2 = −1:
| εr = εr′ + iεr″ | (3) |
The dielectric loss factor εr″ (also called dynamic dielectric coefficient) is obtained by comparing the irradiated microwave energy to the energy that has coupled with the sample. εr″ depends on the dielectric conductivity s and on the frequency f according to eqn. (4):
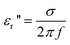 | (4) |
The degree of energy coupling in the reaction system depends on both parameters εr′ and εr″ and is called the dissipation factor D = tan δ, c.f. eqn. (5):
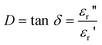 | (5) |
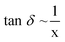 | (6) |
The dissipation factor defines the ability of a medium at a given frequency and temperature to convert electromagnetic energy into heat. It can also be regarded as a measure of the penetration depth x of microwave radiation into a material and is inversely proportional to x (eqn. (6)). According to the definition, the penetration depth is the point where only 37% (1/e) of the initially irradiated microwave power is still present. Penetration depths are only tabulated for a few materials and only for one temperature or for a small range of temperatures.2g,3b Because the penetration depth and the dissipation factor are both strongly dependent on temperature, this fact has to receive special attention for the scaling-up of chemical reactors for industrial applications.
According to the mechanism of energy input (ion conduction or dipole rotation), the dissipation factor additionally depends on other factors. It is directly proportional to the ion concentration, the ion size, the dielectric constant, the microwave frequency, and the viscosity of the reacting medium, cf.2c The dissipation factor of water and most organic solvents decreases with increasing temperature, i.e. the absorption of microwave radiation in water decreases at higher temperatures. In turn, the penetration depth of microwaves increases.
The dielectric coefficients for a number of substances such as organic and inorganic compounds, plastics, ceramics, waxes, glasses, and food are documented in the literature (e.g.27). For common organic compounds, the dependency of the dielectric coefficient on the temperature is known and tabulated.1g,28,29 However, extensive knowledge is missing.
The interaction of electromagnetic radiation with matter is characterised by three different processes: absorption, transmission and reflection.30
Highly dielectric materials lead to a strong absorption of microwaves and consequently to a rapid heating of the medium. This means that εr″ and accordingly tan δ increase and the penetration depth of the microwaves in the medium decreases. Optimal energy absorption is reached in the system.
If microwave radiation is reflected by the material surface, there is no or only a small coupling of energy in the system. The temperature increase in the material is only marginal. This holds true especially for metals with high conductivity. Therefore, microwave devices are internally shielded with a metal surface (Faraday cage) to avoid any leakage of microwave radiation. Because interactions take place even with delimiting surfaces, irradiated energy is dissipated in empty microwave devices so quickly that no relaxation times can be measured.
Non-polar materials exhibit only small interactions with penetrating microwaves and can thus be used as construction materials for reactors. These materials are quartz, pure aluminium oxide (corundum), special glass types, and most common, plastics. Polyethylene and polypropylene have a low softening temperature and can only be used for external reactor parts. Due to their temperature and chemical resistance, fluorocarbon polymers can be used for parts that are in direct contact with hot reaction mixtures. Often combinations of these materials (PTFE + PEEK or PP + PTFE) or composites (e.g. glass fibre-reinforced plastics) are used.
Due to the wide use of microwaves in communication technology, international agreements greatly restrict the frequency domains that can be used for other applications.1f,18 The so-called ISM frequencies are summarised in Table 2. The frequency used in most devices is 2.45 GHz, which is also used in the majority of household microwave ovens.
In the context of these basic principles, the following sections discuss the problems which must be considered in this research field. The microwave radiation is converted into thermal energy and quasi accumulated in the reaction medium. Reactions and processes taking place under the influence of the accumulated thermal energy have thus far always yielded similar results to classical reactions.5,31–34
2.2 Aspects of energy efficiency in microwave assisted reactions
In general, discussion about “energy efficiency” should always relate to comparable parameters. The question whether microwave energy can be used for the activation of chemical reactions more efficiently cannot be answered spontaneously. In ref. 8 and 9 as well as in most of the published work e.g.35–37, investigations were only carried out with very small amounts of reaction mixture in the mmol range, which were then irradiated with comparatively high power (300 to 1000 W). A factor that describes the efficiency of the microwave input was, in our opinion, too rarely included in investigations or discussions.5 Such a discussion was and will be neglected as long as reactions are carried out on a millimolar scale. Considering a scale-up (mmol → mol → kmol), the limitation of this approach becomes obvious.The determination of the energy input (eqn. (7)) and the energy that is required to reach a certain temperature (eqn. (8)) follows simple physical laws:
| Qmw = Pmwt | (7) |
| Qth = mcpΔT | (8) |
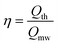 | (9) |
The efficiency factor η can be calculated from both the required and the used energy input (eqn. (9)). The efficiency factor is dimensionless and describes the effectiveness of the conversion of microwave energy into thermal energy. The available microwave power Pmw is determined by microwave device manufacturers according to standardised procedures.38 Therefore, the values calculated from eqn. (7) seem quite reliable. If one checks the values of the microwave power cited in 8 and 9 it becomes obvious that in those investigations the microwave power use was unnecessarily large. For example, 2.5 ml reaction mixture was irradiated with 150–250 W microwave power for 4–9 minutes. At first glance, energies of only 32 and 120 kJ were employed. However, using the thermodynamic data for the materials used in eqn. (9), we find that only 1–1.5 kJ are required to heat the reaction mixture to the reported temperature. The efficiency factor is thus between 0.05 and 0.01 and is far from being an effective conversion of energy. For a more detailed investigation of the energy efficiency, we would like to refer to an article recently published by our group.5 The relations reported in the literature that state the absorbed energy39 and the quotient of the change in temperature and time2c are proportional to the square of the amount of the electric field strength could be verified through our own heating experiments for multiple polar substances in the microwave field. Furthermore, it must be considered that the conversion of electric energy into microwave energy has an efficiency of 0.5 to 0.65.40
The control of the energy input plays an important role in reaching the predefined reaction conditions for the treatment of reaction mixtures in organic chemistry. In household microwave ovens, only time and the power irradiated during this time can be varied as reaction parameters. Thus, the temperature is undetermined and increases steadily during irradiation. A possible but insufficient method to control the temperature is the on- and off-switching of the microwave field within a given time interval.41 In modern laboratory microwave systems, however, computer controls, which allow setting of the attainable temperature or pressure as limiting parameters, are state of the art. This feature is important with regard to safety aspects of handling chemicals and is also crucial for both the reproducibility and the scale-up of reactions. After reaching the preset parameters, the energy input is reduced to a level necessary for keeping the preset values. This power control contributes essentially to the efficiency of the power input by microwaves. The microwave energy proceeds almost free from losses through the reactor walls into the reaction mixture and is then converted into heat. The heat is accumulated in the reaction mixture and remains there since the reactor materials are also good heat insulators.
This control enables the regulation of the temperature in the reaction mixture with a precision of ±1 K and the pressure with ±0.5 bar. With conventional heating, these values cannot be easily reached and represent exceptions. If the reaction parameters are not known, the uncontrolled energy input results in much higher temperatures than those used in conventional reactions. This leads to shorter reaction times and sometimes to higher yields. Those results then nourish the speculations about non-thermal effects. Also, large amounts of reaction mixtures cannot be processed since the explosion and ignition risks are too high. In addition, household microwave ovens are entirely closed systems due to security constraints concerning the complete shielding of electromagnetic radiation. Therefore, they require the use of rather simple laboratory glassware such as open beakers or GC-vials. This complicates the reproducibility of results from such experiments.
In conclusion, it is not surprising that 2.5 ml of reaction mixture are heated very rapidly when irradiated with 250 W microwave power. If one would apply the same controlled concentration of thermal energy to a small vial (e.g. by solar reflectors, Bunsen burner), comparable heating effects and reactions would also result. Comparable power (120–500 W) is used for conventional heating in 250–1000 ml heating mantles, however, for much larger substance quantities and with completely different heat transfer mechanisms, requiring substantially longer heating times.
In our opinion, this point precisely illustrates the advantage of power input by microwaves. High power can be applied to reaction mixtures which are able to absorb microwaves in a controlled and fast manner. The fact that reaction parameters, such as the batch and the vessel size, play an important role will certainly require the education of any synthetic chemists who wishes to employ microwave-assisted reactions.
3 Instrumentation technology
3.1 Aspects of the irradiation method
An often employed argument in the literature (also in 8–11) is the advantage associated with employing a focused energy input when using monomode microwave radiation for chemical reactions compared to using multimode radiation. However, even manufacturers of technical microwave systems do not give consistent definitions of “multimode” and “monomode” devices, cf.5The term “focused” implies the use of optical systems (lenses or mirrors) and is not adequate in this context. Many publications use the term “focused” in an uncritical way without even adding the trademark symbol and without discussing the true meaning of the term. To date, no commercially available microwave system uses a focusing system. Some manufacturers of technical microwaves have registered the term “focused” as a trademark, which has created misunderstandings.42 In a monomode microwave device, the reactor is directly inserted into the waveguide. Microwaves follow the physical laws of electromagnetic radiation. The waveguide is designed in such a way that in the empty waveguide microwaves are reflected in phase. Standing waves result, and the reactor is inserted exactly where a maximum of the electric field was calculated for the dielectric material air. Only the relatively small sample amounts (max. 100 ml, often less) are irradiated at one point from the side. As a result, great inhomogeneities of the electrical field and high temperature differences arise. Furthermore, the insertion of the reactor influences the field geometry and the application of reaction mixtures leads to even further changes, which can not be counteracted by the control mechanisms in the waveguide. New modes (wave kinds) are created by refraction, reflection and interference, which will eventually result in a system with high microwave power density (radiation intensity). This represents a multimode system with an undetermined amount of initial monomode radiation. The amount of monomode radiation apparently depends on numerous parameters such as the reactor size and material, the insertion position in the waveguide, and the constitution and amount of the reaction mixture. Every additional change over time leads to an increase in the amount of multimode radiation. In theory, it makes little sense to distinguish between monomode and multimode radiation. A classification according to their radiation intensity or power density should better characterise today's microwave devices. Table 3 summarises common microwave systems with their important specification parameters.
| a Some other modified or unmodified domestic microwave ovens are used for chemical reactions, e.g. Panasonic NN-S740WA-1200 W, see also ref. 2e and 76. b See also ref. 77. c See also ref. 42b. d See also ref. 78. | ||||
|---|---|---|---|---|
| Manufacturer | Sharpa | Personal Chemistryb | CEMc | MLS/Milestoned |
| Type | domestic MW oven R-220A, | Emrys™ Creator | Discovery™ | ETHOS™ MR |
| Irradiation modus | multimode | monomode | monomode | multimode |
| Max. power | 800 W, pulsed | 300 W, unpulsed | 300 W, unpulsed | 1000 W, pulsed or unpulsed |
| Cavity volume | 15.7 L | <1 L | <1 L | 42.8 L |
| Maximum power density in empty cavity | around 50 W L−1 | >300 W L−1 | >300 W L−1 | around 23 W L−1 |
| Reaction scale | max. 100 g in dry reactions | <20 g | <50 g | up to 3000 g depending on reactor |
The second type of commercially available microwave system is multimode systems. In these devices, the radiation produced by the magnetron is directed through a waveguide and a mechanical field distributor in a rather large volume (microwave cavity). In the cavity, radiation is in general homogeneously distributed, thus avoiding the formation of standing waves. While household microwave ovens exclusively operate with pulsed microwave radiation, technical systems also allow the use of continuous (unpulsed) irradiation. In the pulsed operation mode, pulses with the maximum available power (800–1000 W) are applied according to the preset irradiation power and time. In the unpulsed mode, the actual preset power is applied (Fig. 3, designed by W. Lautenschläger, MLS GmbH, Leutkirch, Germany).
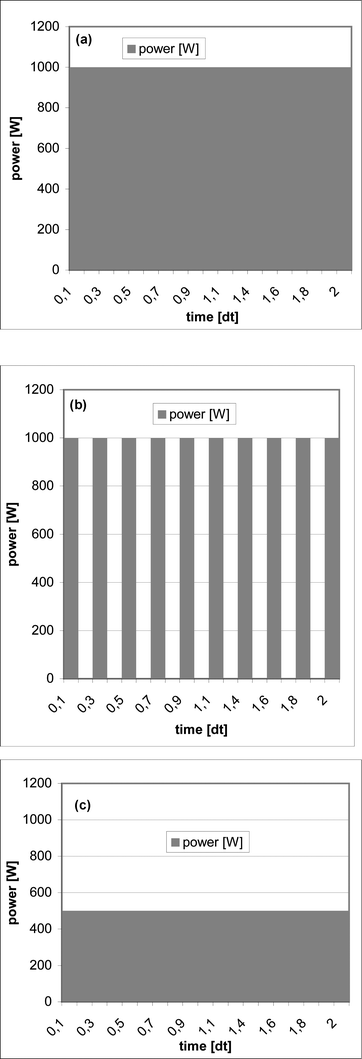 | ||
| Fig. 3 Microwave power at work with pulsed and unpulsed power supply unit, maximum power of the discussed system: 1000 W (a) Irradiation of 1000 W. No differences are found between pulsed and unpulsed power supply units. (b) Irradiation of 500 W with pulsed power supply units. Irradiated with full power (1000 W) in 50% of predetermined time. (c) Irradiation of 500 W with unpulsed power supply units. Irradiated with 50% power in the complete predetermined time. | ||
Working with unpulsed microwave radiation exhibits advantages for refluxing, distilling and for photoreactions in the microwave field (e.g.4). Sensitive substances might not be overheated. The disadvantage of these unpulsed power supplies in comparison to conventional systems is their higher technical complexity. However, since the use of the full microwave power5 did not seem advantageous for most of the investigated reaction, the use of unpulsed power supplies will result in advantages in the long run, also with respect to energy efficiency.
At this point, we would like to discuss the term “penetration depth” in more detail. The microwave cavity of the largest available synthetic microwave system has a volume of approximately 100 L and allows for reactions on the pilot plant-scale. Microwaves penetrate from all sides into the sample and lead to a mostly homogeneous energy input, which is additionally improved by stirring the reaction mixture. Considering the penetration depth of microwaves in water, 1.4 cm at 25 °C and 5.7 cm at 95 °C, the reactor dimensions are limited.25 Currently a more precise prediction for organic solvents is impossible due to a lack of data. However, since water has a comparatively high dielectric coefficient, higher penetration depths can be expected at ambient temperature for the majority of substances used in organic synthesis.
Literature5,32–34 reveals that for a series of investigations when other reaction parameters were kept constant (e.g. temperature, batch size, mol ratio), the irradiation method (monomode or multimode) did not influence the result of the experiment. Examples include the enzyme-catalysed transesterification of ethyl acetate or vinyl ester with racemic alcohols for enantiomeric separation,32 the Hantzsch reaction of ethyl acetoacetate with formaldehyde and ammonia, the Knoevenagel reaction of salicylaldehyde with ethyl acetoacetate (Scheme 1),5 hydrolysis of benzamide33 and examples of the palladium-catalysed C–C-coupling.34
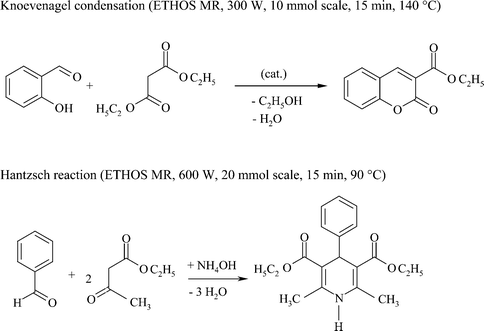 | ||
| Scheme 1 | ||
3.2 Aspects of the temperature measurement in the microwave field
Commonly employed mercury thermometers cannot be used for temperature measurements in the microwave field because they absorb microwaves (act as antenna). When the mercury heats up, it builds a self-potential, which creates feedback with the magnetron or the cavity walls. This can lead to a spark discharge (compensation of potential) that destroys the thermometer.Due to a lack of alternatives, temperature measurement was often neglected during the first years of microwave-assisted reactions, e.g.2e,13,14 The obtained results were compared with data in literature, which sometimes lead to incorrect estimations of the capability of microwave radiation for the activation of chemical reactions. Due to the comparatively high costs, new temperature measurement systems were only slowly introduced. These systems allowed for the temperature measurement directly inside the microwave field or the reaction mixture. Three essential methods for the measurement of temperature in the presence of microwaves exist:
(i) shielded thermocouples
(ii) IR-sensors
(iii) fibre optics
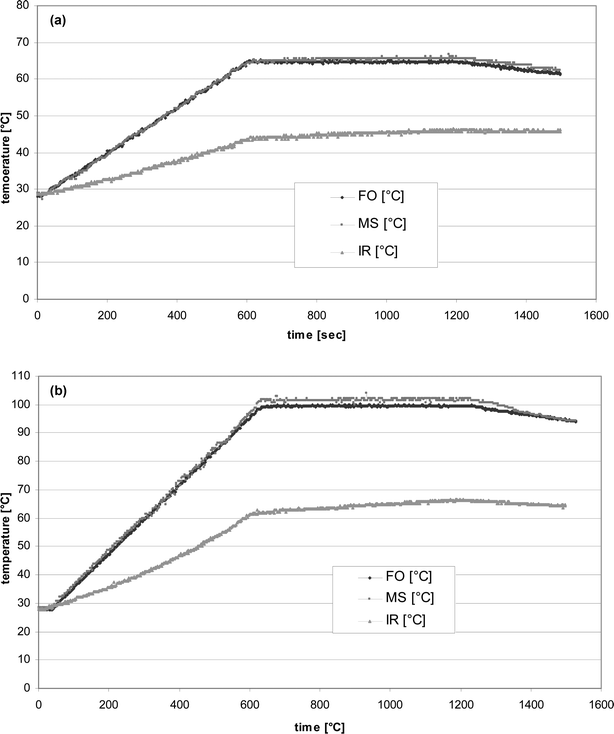 | ||
| Fig. 4 Comparison of temperature measurement with different sensors. Irradiation of 500 g water with 500 W microwave power (ETHOS 1600 *, reflux apparatus) (a) Temperature range: 28 °C up to 65 °C. (b) Temperature range: 28 °C up to 100 °C. *The fibre optic sensor (FO) and the metal sensor (MS) are placed directly into the stirred medium and the precision of measurement for the FO-sensor and metal sensor are ±2 K. The IR-sensor measured the temperature on the outer surface of the reactor. The temperature increase is controlled by a programmed predetermined ramp step and controlled with FO-sensor. | ||
The investigated measurements with three different measurement methods exhibit a margin of error of ΔT = 30 K, especially when reaction mixtures were heated quickly and when reaction times were short (<20 min). The IR-sensor always registers lower temperatures. This discrepancy explains many speculations about microwave effects in systems where only IR-sensors were employed.20,21 For example, if an IR-sensor reads 220 °C for a microwave-assisted reaction (cf.20 in8) and the same reaction is reproduced at exactly 220 °C when measured by a thermocouple or a mercury thermometer under conventional heating methods, any difference in the course of the reaction could stem from more than one parameter (temperature measurement or energy input). A questionable concept for temperature measurement and reaction processing using IR-sensors is propagated by the CEM Corp. under the name PowerMAX™.42 In this concept, the reactor wall is cooled directly with a strong air stream and at the same time the power input of the microwave system is increased. The temperature at the reactor wall (measured with an IR-sensor) remains constant because the heat flux through the glass wall remains constant. The cooled air stream is able to keep the temperature of the reactor wall at the preset point. The temperature rise inside the reactor is undetected, and thus higher conversions for sample reactions can be obtained. Additionally, this cooling system contributes to energy dissipation and can incorrectly lead the operator to the conclusion that higher microwave power leads to higher conversions. The same result is obtained when one works without the intensified cooling by setting the temperature higher and applying regular microwave energy, cf.43
The measuring range of IR-Sensors currently used is between −40 and +1000 °C. Such sensors are used by all manufacturers of technical microwave systems and are fairly widespread. For devices from CEM and Personal Chemistry, the IR-sensor is the lead-sensor and controls the power input. MLS/Milestone uses the IR-sensors in several systems for secondary measurements that explicitly control the temperature on the reactor surface.
Although there are disadvantages in the operating range and a reduced mechanical stability in fibre-optical sensors, they still have a broad range of applications for temperature measurement in the microwave field. Furthermore due to the low volume requirements, the sensors can also be applied to small scale reactions. At present, the commercial availability of fibre-optical measurement systems with a constant sensor quality remains a problem. The precision of the temperature is crucial for the reproducibility of the experiments and the comparison with conventional reactions.
It must be concluded that the problem of temperature measurement within the microwave field is mostly solved. However, a breakthrough in the precision of the working methods is still sought and until implementation there is still room for more speculations or discussions about non-thermal effects during a microwave reaction.19–23,42 The possibility that all microwave effects found are due to incorrect temperature measurements and invalid comparisons with conventional reactions cannot be ruled out. The proof of this statement, however, requires a large number of reproducible experiments.
4 Applications – experiments – comparisons
4.1 Aspects of microwave-assisted synthesis as an interdisciplinary research field
The questions on generalisation, reproducibility and scale-up of microwave assisted reactions will always be centred on the reaction conditions. Additionally in microwave-assisted reactions, the medium plays a much more important role than in classical reactions. Also, the polarity of all components in the reaction mixture determines the absorption of microwave power. Since dielectric constants (static and dynamic) are only known for a few compounds, further complexity arises and contact with neighbouring disciplines, for example with electrical engineering in this case, is required in order to further study and ultimately, to better understand the reaction.The concept of a “Comprehensive Chemistry” is not useful in the case of microwave-assisted reactions. Compared to conventional reactions, the reaction engineering and technical parameters play a much larger role in microwave-assisted reactions. Therefore, an improvement of the description of reaction parameters is crucial. These considerations lead us to develop a draft for a general experimental protocol for microwave-assisted reactions and processes. Its introduction and application in the sense of sustainability shall be presented later for some examples.
The batch sizes, the energy input, and especially the method of the temperature measurement are rarely described correctly or completely. Thus it is impossible to place a value on the described reaction and its reproducibility.
As a result of long-lasting effort between the microwave system manufacturers and the chemical engineers, a concept for the transfer of conventional reactions into the microwave field, which attempts to find solutions through “scale-down” and “numbering-up” approaches as well as through “scale-up” concepts (Fig. 5), has been developed.
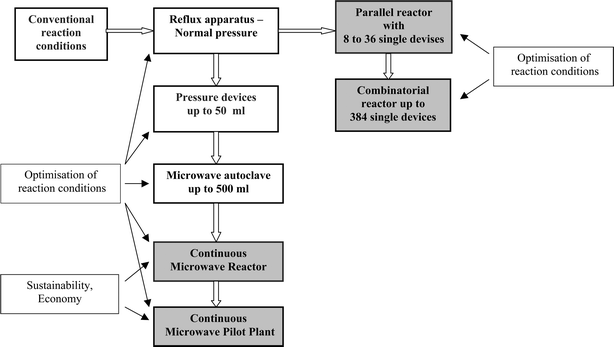 | ||
| Fig. 5 Development concept for microwave assisted chemical reactions and processes. | ||
The following applications demonstrate the realisability of the concept and some possibilities of the developed complex microwave system. Some problems of scaling-up are discussed in Section 4.3.
4.2 Application examples
Microwave technology provides an alternative source of energy that should be well suited for preparative extractions.45 Not only is it possible to introduce energy quickly into the reaction system, but also the extract and the energy can be quickly removed from the system. After a series of preliminary experiments for analytical extractions,46 the HEF 270 extraction system with a 6-fold segmented rotor was developed in cooperation with MLS.47 This extraction system enables the heating of the extraction mixture in a controlled fashion. For example, the separation of trimyristine directly from the nutmeg powder was achievable.
With this system, even heat sensitive natural material can be isolated since the residence time at the elevated temperatures is very short (approx. 1 min). This simple and easy-to-operate system was used to study aspects of the non-classical energy input (in this case: microwaves) for chemical extractions and processes in the education of graduate chemistry students.48
As an introductory example, the extraction of trimyristine from nutmeg powder was chosen (Fig. 6).49 Common unground nutmeg nuts contain between 10% and 40% of extractable substances. The main constituent of those substances is a triglyceride consisting of 90% myristic acid (saturated C14 carboxylic acid). Due to its solubility, trimyristine can be easily isolated by hot extraction with ethanol.
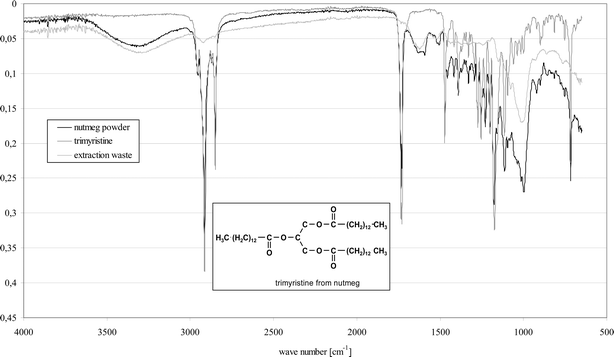 | ||
| Fig. 6 Comparison of FTIR-spectra (ATR, TravelIR, Perkin Elmer Instruments, Sheldon, CT) of nutmeg powder, trimyristine and extraction waste. | ||
Comparative investigations were performed with Soxhlet apparatuses with additional attention paid to the choice of solvent, completeness of the extraction and the required amount of energy. While the conventional extraction was performed with 10 g nutmeg powder at approximately 80 °C and with a solvent volume of 300 ml over the course of 4 to 6 hours, in the microwave field, the extraction of 3 × 3.3 g nutmeg powder, each with 80 ml ethanol at 100 °C, only required 10 to 15 minutes of extraction time. The yields of trimyristine in the microwave experiments are approximately 10% higher than those under conventional techniques with comparable batches of raw product. With the microwave-assisted conditions, an almost quantitative extraction was obtained. Comparison of IR spectra of the raw product, trimyristine and the extraction residue (Fig. 6) from a second extraction step showed this high conversion.
A proposed protocol for the microwave-assisted extraction experimental is summarised in Table 3.
This protocol allows for an easy implementation of a non-classical experiment into the curriculum of chemistry students. The starting materials are known and the concept is practical. Students without any previous knowledge of microwave techniques and processes could, after a short introduction, operate the microwave system including the extraction rotor and perform the extraction of a natural product.
The reaction of fumaric acid diethyl ester with anthracene to the respective Diels–Alder adduct is a well-investigated1e and comparatively simple reaction. It proceeds in high yield under conventional conditions in the presence of equimolar amounts of anhydrous aluminium chloride as an activator (Scheme 2).51 If no activator is used, heating for several days in dioxane or for several hours in p-xylene is required to achieve high yields.52
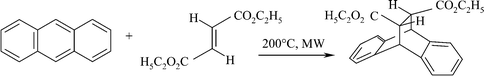 | ||
| Scheme 2 Diels–Alder reaction (cf.Table 4, entry b). | ||
Surprisingly, results that have been described once with defined reaction conditions are rarely investigated further and are only revised in very few cases.48 Moreover, they are usually carried out as described in the literature.
If one takes a closer look at the selection of a solvent, this is mostly determined by the desired reaction temperature or perhaps the viscosity of the reaction mixture. Normally, especially with respect to scale-up, diluting of the reaction mixture is undesirable. For reactions at elevated temperatures, the solvent is often used in order to keep the reaction temperature constant for an extended period of time. If another control mechanism exists, the use of a solvent can perhaps be avoided. Without the solvent, the temperature range up to the boiling point of the reactants can be exploited. This in turn brings into question the necessity of an activator or catalyst.
The reaction represented in Scheme 3 was performed in the microwave field after extensive preliminary tests. As a result of the comparative experiments with the conventional reaction conditions, it was shown that without the solvent, reaction temperatures could easily be set to 150–250 °C. This led to a reduction of the reaction time to 10 minutes and rendered the activator superfluous. The temperature was reached within a few seconds for a 1 mol batch and kept constant with the modern automatic control technology of the technical microwave systems. Thus, the reaction conditions changed drastically: Two auxiliaries were not required and no aqueous work-up was needed. With these measures, a previously published classical reaction was improved and now the new possibilities of modern reaction engineering can be implemented. Reaction conditions are summarised in Table 4.
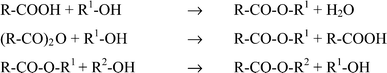 | ||
| Scheme 3 Possibilities of esterification. | ||
| 1. General data | Microwave–assisted extraction: trimyristine from nutmeg powder | Solvent free – Diels–Alder reaction: anthracene with fumaric acid diethyl ester |
|---|---|---|
| Type of microwave system | ETHOS 1600; | ETHOS MR |
| Manufacturer | MLS GmbH Leutkirch | MLS GmbH Leutkirch |
| Construction year | 2000 | 1998 |
| 2. System description | ||
| Cavity volume | 42 L | 42 L |
| Max. microwave power | 1000 W | 1000 W |
| Characteristic of magnetron | 2 industry magnetrons, power drop down from 800 to 500 W. | 2 industry magnetrons, power drop down from 800 to 500 W. |
| Power dosage | 10 W steps | 10 W steps |
| Irradiation modus | multimode | multimode |
| Microwave irradiation | unpulsed | unpulsed |
| 3. Reactor and program | ||
| Type of reactor | HEF 270, segment rotor, 3 segments | reflux apparatus, 100 ml flask |
| Temperature measurement | fibre optic in one segment | fibre optic |
| Safety equipment | MW-leak sensor | MW-leak sensor |
| Control program | 5 min/500 W/up to 100 °C – 10 min/400 W/at 100 °C | 15 min/700W/up to 220 °C – 5 min/900 W/up to 250 °C – 15 min/900 W/at 250 °C |
| Max. temperature | 110 °C | 220–250 °C |
| Max. pressure | 4–5 bar | 1 bar |
| Average power | 197 W | 522 W |
| Energy entry | 175 kJ (kWs) | 1112 kJ (kWs) |
| Stirring in reactor | magnetic stirring bar, 14 mm | magnetic stirring bar, 30 mm cross form |
| 4. Chemical data | ||
| Scheduled quantity | 10 g nutmeg powder | 0.1 mol |
| Amount and ratio of all components | 3.3 g nutmeg powder in each rotor segment | anthracene: 17.8 g, fumaric acid ethyl ester: 20.7 g (20% excess) |
| Solvent | 80 ml ethanol in each rotor segment | |
| Dosage of reaction compounds | assembling of segments following construction manual46 before installation of rotor in the microwave system. | mixing of components before installation of flask in microwave system. |
| Reaction behaviour | pressure extraction | solvent-free reaction at boiling point of fumaric acid ethyl ester (219 °C), after reaction time at 250 °C |
| Cooling method | Extract was taken hot. | air cooling |
| Cooling time | 20 min | |
| Work-up | cooling of extract, filtration in vacuum, drying on air, transesterification of triglycerides | stirring with petrolether, washing, vacuum filtration |
| Yield | depended of quality of nutmeg powder | 92% |
| Analyses | mp., IR, NMR, GC of FAME | mp., GC, NMR |
(i) EXPLORER-System (CEM) and EMRYS-Systems (Personal Chemistry)
(ii) ETHOS (MLS/Milestone) and Multiwave 3000 (PAAR)
(i) The first method allows for the use of small microwave cavities with high microwave density, for irradiation of solely the reactor (e.g. GC vial) and volumes of up to 50 ml. This approach is used by the companies CEM and Personal Chemistry (cf.Table 3). Short reaction times under controlled reaction conditions (temperature measurement via IR-sensor) are used for a step-by-step processing of a large number of assays.
(ii) The ETHOS system from the company MLS/Milestone takes a different approach which allows for the simultaneous irradiation of several assays (sample volumes: 1–100 ml) in a larger microwave cavity under identical reaction conditions. For this purpose, some rotor reactor racks that can accommodate up to 192 assays were developed in cooperation between the company MLS GmbH and the ITUC of Friedrich Schiller University of Jena. Test reactions performed in this reactor set-up have been published.57 It is noteworthy that these tests confirmed that the reaction time itself is not the rate-determining step for combinatorial chemistry in the microwave field. It is rather the data management, sample preparation, work-up and sample analyses that consume the most time.
4.3 Aspects of process development
A common requirement associated with the introduction of a new technology is the possibility to scale-up the respective process, first to a pilot plant-scale and eventually to the production scale.The aim of using microwave processing is to accelerate reactions in order to avoid disadvantageous reaction parameters (i.e. long reaction times, secondary reaction time, solvent use, excess components etc.). A further goal for process improvement is to transfer a batch operation to a continuous operation after they have undergone a process analysis. For this purpose, usually the first step is to repeat the known reaction conditions used in the conventional reactions in the microwave field. Often a similar experimental setup is used (reflux apparatus). Starting from this point, all conventional reaction conditions must be re-evaluated. The introduction of a new technology in organic technical synthesis allows for the questioning of old preparation protocols. It would be advantageous to produce a check list for each reaction that critically questions the known synthetic protocols, analyses them, and provides potential new solutions. When considering process development a variety of parameters should be addressed. A list of questions that could be asked follows:
• What is required temperature for the reaction?
• What is the influence of the temperature?
• Are the compounds in the reaction mixture thermally stable?
• Is the reaction exothermic or endothermic; what is the energy balance?
• Do secondary reactions occur? Why?
• Is a solvent required? Why?
• How is the work-up? Do the work-up processes require (heat) energy?
• Is a catalyst required? Which catalyst and in what quantity?
• Can the catalyst be recycled? Is the recycling advantageous?
• Which reaction conditions influence each other?
• Is the stoichiometry optimum?
• What waste materials and/or by-products form and how can they be handled?
• Do toxicological/ecotoxicological data exist?
This list can be extended on the basis of the rules for sustainable development10,58 in all areas and is not meant to be an exhaustive list. Microwave technology allows for the investigation of these questions with the present technology by changing reaction parameters and checking old reaction protocols.
Microwave systems that are currently commercially available were initially developed for chemical decomposition (complete mineralization as sample preparation for atomic adsorption spectroscopy). This development limited the size of the reactors that could potentially be used for chemical synthesis. With operating volumes of 25–50 ml, or in rare cases 250 ml, reactions on the 10–50 mmol scale could be performed. Relating to this, the concept of dry reactions has been reported,5 and cited in the literature.
The development of microwave systems for further applications in organic chemistry is going in several directions: one trend is the development of small devices or devices that are tailored to a special application. The small devices (cf.Table 3) allow for the reaction of mmol-amounts in a short time (several minutes) with comparatively high power input. These devices posses a small microwave cavity (≤1 L) and have a reactor installed directly in the waveguide, often only small and closed vessels similar to GC-vials can be used. These systems are advantageous for organic chemists if only a yes-or-no-answer with respect to the experimental result is expected (i.e. for screening conditions). If the investigatory scope is extended to questions about the reproducibility, the reaction kinetics, or the increase in the reaction scale up to 0.1 mol product (factor 100), such devices will fail. This product line was developed by three companies:
– Personal Chemistry (Sweden) – automated products of the EMRYS-systems
– Prolabo (F) (no longer existing) – “real” monomode systems (see section 3.1) of the “Synthewave”-series
– CEM (USA) – “Discovery”-series. CEM also produces a wide range of multimode devices, which are mainly used for sample preparation (digestion, drying, ashing) and sometimes also for carrying out organic syntheses.59,60
However, according to the previous explanations, the EMRYS and the Discovery systems do not represent real monomode systems, but rather are multimode systems with a high power density.61
The second development direction is the design of different reactor kits that can be integrated into the same basic system (ETHOS system, MLS/Milestone). This system allows for the realisation of a concept (see Fig. 5) for the comparative transition of classical thermal reactions into the microwave field. The use of different reactors for different requirements and applications allows for a flexible reaction engineering in which reaction parameters can be precisely documented. Through the exact reproduction of conventional conditions, it is possible to simultaneously compare classical and microwave-assisted reactions. With this construction kit, reactions from the mmol-scale to the mol-scale can be performed. Furthermore, the transition from a batch operation to a continuous operation is also imaginable. This has already been described for some reaction types.62
Derived from the basic model, a robust beginner system (PRAKTIKA, MLS GmbH) is now available with simple measurement technology that allows for easy integration of microwave-assisted reactions into laboratory classes.
Further, a pilot plant device was derived from the base mode in which first studies on the real scale-up were performed.63 The ETHOS 4000/4001 devices can already process reaction mixtures of 5–10 kg per hour and are thus already suitable for the production of high-priced fine chemicals, e.g. pharmaceuticals.
The goal of all these investigations was to obtain a holistic view and to question all reaction parameters employed so far in order to discover new unconventional ways for carrying out long-known reactions. Therefore, the description of all reaction parameter is absolutely necessary.
4.4 Application example – esterification of linalool with carboxylic acid anhydride
Various esterification reactions were repeatedly performed in the microwave field (Scheme 3).64 It was found that if all reaction conditions were similar between the conventional and microwave-assisted reactions, no differences in the reaction kinetics were observed.65The esterification of tertiary alcohols is a well established process that poses significant problems,66 especially when other functional groups are present and when the process is to be transferred from the laboratory-scale into the pilot plant-scale and the production-scale.
In the realm of process design, the use of microwave energy for the esterification of linalool with different carboxylic acid anhydrides was investigated (Scheme 4).
 | ||
| Scheme 4 Formation of linalooyl ester (cf.Table 6, entry a and b). | ||
First, the parameters for the batch process were analysed, and then in a second development step, the reaction was transferred into a continuous process. Table 5 summarises reaction parameters for the continuous esterification (1. step in Table 5) of linalool with carboxylic acid anhydrides in the continuous microwave system ETHOS contFLOW.
| Reaction duration/min | Yield of linalyl propionate (GC, area-%) |
|---|---|
| 42 | 49.7 |
| 120 | 52.2 |
| 215 | 50.8 |
| Average yield | 51.1 |
With these parameters, orienting experiments were carried out in the pilot plant microwave system ETHOS PILOT 4000. After a short starting-up phase, the reactions on the 25 kg-scale showed relatively constant conversion and product composition through the experiment (Table 5).
Parallel to the reaction, improvement of the whole process, especially with respect to the thermal steps in the work-up procedure and in the recycling of the secondary products, using microwave energy was made. For this purpose, the reaction mixture (conversion 50–60%) was submitted to a reactive distillation,67,68 in which the carboxylic acids formed were distilled under vacuum at temperatures of 10–20 °C below the actual reaction temperature (2. step in Table 5). Thus, the reaction equilibrium could be completely shifted in favour of reaction products. The losses of linalool (dehydration to olefins, rearrangement to nerol and geraniol, and formation of isomeric esters) were less than 10%. Table 6 provides a proposal for the protocol for all steps in the continuous lab scale esterification.
| 1. General data | 1. step: continuous reaction | 2. step: reactive distillation | 3. step: reactive distillation – reaction of carboxylic acid with acetic acid anhydride |
|---|---|---|---|
| Microwave system | ETHOS contFLOW, | ETHOS MR | ETHOS MR |
| Manufacturer | MLS GmbH Leutkirch | MLS GmbH Leutkirch | MLS GmbH Leutkirch |
| Construction year | 1998 | 1997 | 1997 |
| 2. System description | |||
| Cavity volume | 42 L | 42 L | 42 L |
| Max. microwave power | 1000 W | 1000 W | 1000 W |
| Characteristic of magnetron | 2 industrial magnetrons; Power drop down from 800 to 500 W. | ||
| Power dosage | 10 W steps | 10 W steps | 10 W steps |
| Irradiation modus | multimode | multimode | multimode |
| Microwave irradiation | unpulsed | unpulsed | unpulsed |
| 3. Reactor & program | |||
| Type of reactor | contFLOW reactor; pump: KM281, Aldos Eichler GmbH | rectification apparatus with 2000 ml flask | rectification apparatus with 2000 ml flask |
| Temperature measurement | shielded thermocouple, | fibre optic | fibre optic |
| Safety equipment | MW leak sensor | ||
| Control program | 10 min/500 W/up to 150 °C – 300 min/500 W/at 150 °C | 5 min/750 W/up to 135 °C – stepwise (1 K) to 142 °C depending on distillation behaviour/500 W/time: 600 min | 5 min/750 W/up to 125 °C – stepwise (1 K) to 130 °C depending on distillation behaviour/500 W/time: 480 min |
| Maximum temperature | 160 °C | 145 °C | 135 °C |
| Maximum pressure | 15 bar | ||
| Minimum pressure | 80 mbar | 100 mbar | |
| Average power | 500 W, | 432 W, | 482 W |
| Energy entry | 4500 kJ (kWs) | 10388 kJ (kWs) | 9328 kJ (kWs) |
| Stirring in reactor | magnetic stirring bar, 30 mm cross form | magnetic stirring bar, 30 mm cross form | |
| 4. Chemical data | |||
| Scheduled quantity | 10 mol | 1300 ml reaction mixture | 1300 ml reaction mixture |
| Amount/ratio of components | linalool ∶ carbon acid anhydride = 1 ∶ 1.5; 10 ∶ 15 mol | reaction mixture from 1. step | distillate from 2. step with equimolecular amounts of acetic acid anhydride |
| Catalyst | potassium carboxylate, 18 mmol/mol linalool | ||
| Dosage of reaction compounds | mixed compounds pumped through the reactor | components mixed before placing flask in microwave system | |
| Reaction behaviour | continuous reaction, residence time: 12 min | distillation of carbonic acid, carbonic acid anhydride and small amounts of terpene olefins from 1. step | distillation of acetic acid out of the reaction mixture |
| Cooling method | direct cooling | air cooling after end of distillation | air cooling after end of distillation |
| Work-up | after filtration of catalyst, washing with sodium carbonate solution and vacuum distillation | vacuum distillation of the raw carbonic acid anhydride | |
| Yield | conversion around 50% | overall yield 85% linalyl carboxylate | 95% of carbonic acid anhydride |
| Analyses | GC | GC, GC-MS, NMR | GC, GC-MS, NMR |
The investigations of the reactive distillation show that distillation processes especially benefit from the use of microwave energy. The temperature measurement directly at the bottom of the distillation and the previously discussed advantages of the inversed heat flux in the microwave field, prevent overheating of the vessel walls, which may arise under external heating. Thus higher yields, suppression of decomposition reactions, and a longer stability during distillation result. The elimination of decomposition reactions is of great importance, for example in the fabrication of perfumes and is essential for improvements in the end product quality, since foreign odours can disturb the olfactory system. Besides the reactive distillation for the quantitative esterification of linalool, another reactive distillation was used for recycling the carboxylic acids to anhydrides (Scheme 5).
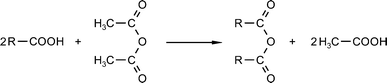 | ||
| Scheme 5 Formation of acid anhydride (cf.Table 6, entry c). | ||
Mixtures of higher carboxylic acids and acetic anhydride were heated in the microwave field to 120–125 °C (b. p. of acetic acid: 118 °C) and the formed acetic acid was distilled from the reaction under vacuum.
This method is suitable for producing larger amounts of carboxylic acid anhydrides (up to 2 kg), which are otherwise difficult to obtain from commercial suppliers. A comparatively short reaction time of 4–6 hours (3. step in Table 6) was used. Since acetic anhydride is a low-cost starting material and anhydrides of higher carboxylic acids are interesting intermediate products for the perfume industry, this procedure could be of economic interest.
Using an appropriate distillation column, the mixed anhydrides that form as intermediate products do not disturb the work-up of the carboxylic acid anhydride that remains in the distillation bottom. For these power input parameters, the microwave reaction system (ETHOS MR) was able to keep up to 2 L of reaction mixture at 135 °C, and thus only the capacity of the reaction column determines the effectiveness of the process.
It has to be noted, however, that the vacuum precision distillation of the perfume esters and the higher carboxylic acid anhydrides cannot be performed in the microwave field for safety reasons. At pressures below 100 mbar, microwave plasma might ignite and therefore it is too dangerous to perform reactions and processes in this domain.69
Another example for the beneficial application of microwaves is the acylation of tocopherols70 to the main commercial form of vitamin E, (all-rac)-α-tocopheryl acetate. The acylation reactions, more particularly the synthesis of tocopheryl acetate, can be carried out in the absence or presence of a catalyst and solvent-free. Excellent yields and selectivities could be achieved (>99% yield, total conversion of starting material). The reaction can be carried out continuously in kg scale and is of great commercial interest.
5 Conclusions
“Simple heating with microwaves” has become a common laboratory practice for many preparative chemists. Microwave systems with integrated on-line control guarantee safe operation and open a vast field of applications, even on the technical scale. In this context, it seems worthwhile to critically assess all reaction parameters for syntheses and separations and to coordinate them with each other. In other words, besides the obvious need to learn more about microwave-assisted reactions, there are also promising challenges ahead.For technical applications and implementation of a new method, it is necessary to have equipment available. For application in a synthetic pathway not only criteria like E-factor71 or atom economy are important, the equipment factor also plays an important role:72
| Implementation = Atom Economy × (weighted) E-Factor × Equipment Factor |
For example, if the equipment is available around the world without any restrictions, the factor is 1. In the case of microwaves the factor is around 0.3 because for large scale industrial production (several 1000 tons), currently no equipment exists.
For further chemical applications of alternative energy entries in reactions systems the development of new apparatus and improvement in reactor design is strictly recommended.
6 Symbols
λ 0 wavelength at vacuum conditions [cm]c speed of light [2.998 × 1010 cm s−1]
f frequency [Hz]
ε r relative dielectric coefficient
C capacity [F]
C 0 capacity under vacuum conditions [F]
ε r″ dielectric loss factor (dynamic dielectric coefficient)
σ dielectric conductivity
D dissipitation factor (= tan δ)
x penetration depth [cm]
Q mw microwave power input [kWs]
P mw microwave power [W]
t time [sec]
Q th required thermal energy [kJ = kWs]
T Temperature [ °C = K + 273.15]
ΔT temperature difference [K]
c p specific heat capacity [J K−1 kg−1]
m mass [kg]
η efficiency
Acknowledgements
The authors would like to thank Mr. W. Lautenschläger and Mr. F. Visinoni (MLS GmbH Leutkirch, Germany, and Milestone srl., Sorisole, BG, Italy) for technical cooperation and discussions in development of the a. m. reactor systems, and Mrs. A. Tied, Mr. R. Trotzki for technical assistance. B. O. thanks the Fonds der Chemischen Industrie for financial support. Furthermore we thank Mr. W. Zinsser (Zinsser Analytik GmbH, Frankfurt/Main, Germany) for timely providing a LISSY®-equipment and Mr. A. Görig (BFF GmbH Miltitz, Germany) for assistance in the AiF-Project No: FUEGO-0037801L8.References and notes
-
(a) R. N. Gedye, F. E. Smith and K. Ch. Westaway, Can. J. Chem., 1988, 66, 17 CAS
; (b) R. A. Abramovitch, Org. Prep. Proc. Int., 1991, 23, 685
; (c) D. M. P. Mingos and D. R. Baghurst, Chem. Soc. Rev., 1991, 20, 1 RSC
; (d) A. G. Whittaker and D. M. P. Mingos, J. Microwave Power Electromagn. Energy, 1994, 29, 195
; (e) S. Caddick, Tetrahedron, 1995, 51, 10403 CrossRef
; (f) Ch. R. Strauss and R. W. Trainor, Aust. J. Chem., 1995, 48, 1665 CAS
; (g) K. C. Westaway and R. N. Gedye, J. Microwave Power Electromagn. Energy, 1995, 30, 219
.
-
(a) A. K. Bose, B. K. Banik, N. Lavlinskaja, M. Jayaraman and M. S. Manhas, CHEMTECH, 1997, 9, 18
; (b) S. A. Galema, Chem. Soc. Rev., 1997, 26, 233 RSC
; (c) C. Gabriel, S. Gabriel, E. H. Grant, B. S. J. Halstead and D. M. P. Mingos, Chem. Soc. Rev., 1998, 27, 213 RSC
; (d) R. N. Gedye and J. B. Wei, Can. J. Chem., 1998, 76, 525 CrossRef CAS
; (e) R. S. Varma, Green Chem., 1999, 1, 43 RSC
; (f) N. Elander, J. R. Jones, S.-Y. Lu and S. Stone-Elander, Chem. Soc. Rev., 2000, 29, 239 RSC
; (g) P. Lidström, J. Tieney, B. Wathey and J. Westmann, Tetrahedron, 2001, 57, 9225 CrossRef CAS
; (h) F. Chemat and E. Esveld, Chem. Eng. Technol., 2001, 24, 735 CrossRef CAS
; (i) M. Larhed, Ch. Moberg and A. Hallberg, Acc. Chem. Res., 2002, 35, 717 CrossRef CAS
.
-
(a)
D. M. P. Mingos and A. G. Whittaker, “Microwave Dielectric Heating Effects in Chemical Synthesis”, in Chemistry Under Extreme or Non-classical Conditions, ed. R. van Eldik and C. D. Hubbard, John Wiley & Sons and Spektrum Akademischer Verlag Co-Publication, New York and Heidelberg 1997, p. 479 Search PubMed
; (b) D. M. P. Mingos and D. R. Baghurst, “Applications of Microwave Dielectric Heating Effects to Synthetic Problems in Chemistry”, in Microwave Enhanced Chemistry, ed. H. M. Kingston and St. J. Haswell, ACS, Washington, DC, 1997, p. 3 Search PubMed
; (c) G. Majetich and K. Wheless, “Microwave Heating in Organic Chemistry – An Update”, in Microwave Enhanced Chemistry, ed. H. M. Kingston and St. J. Haswell, ACS, Washington, DC, 1997, p. 455 Search PubMed
; (d) R. S. Varma, “Environmentally benign organic transformations using microwave irradiation under solvent-free conditions”, in Green Chemistry – Challenging Perspectives, ed. P. Tundo and P. Anastas, Oxford University Press, Oxford, UK, 2000, p. 221 Search PubMed
, see also R. S. Varma, Advances in Green Chemistry: Chemical Syntheses Using Microwave Irradiation, AZREFI Special Publication No 008, Bangalore, India, 2002 (address for a free copy: E-mail: azrefi@astrazeneca.com) Search PubMed
; (e) Microwaves in Organic Synthesis, ed. A. Loupy, Wiley-VCH, Weinheim, 2002 Search PubMed
; (f) B. H. Hayes, Microwave Synthesis, CEM Publishing, Matthews, NC, 2002 Search PubMed
.
-
(a) M. Nüchter, B. Ondruschka, A. Jungnickel and U. Müller, J. Phys. Org. Chem., 2000, 13, 579 CrossRef
; (b) M. Nüchter, B. Ondruschka and W. Lautenschläger, Synth. Commun., 2001, 31, 1277 CrossRef CAS
; (c) R. Trotzki, M. Nüchter and B. Ondruschka, Green Chem., 2003, 5, 285 RSC
.
- M. Nüchter, U. Müller, B. Ondruschka, A. Tied and W. Lautenschläger, Chem. Ing. Tech., 2002, 74, 910
, engl. version: M. Nüchter, U. Müller, B. Ondruschka, A. Tied and W. Lautenschläger, Chem. Eng. Technol., 2003, 26, 1207 Search PubMed
.
- A. Loupy, A. Petit, J. Hamelin, F. Texier-Boullet, P. Jacquault and D. Mathe, Synthesis, 1998, 1213 CrossRef CAS
.
-
(a) R. Laurent, A. Laporterie, J. Dubac, J. Berlan, S. Lefeuvre and M. Audhuy, J. Org. Chem., 1992, 57, 7099 CrossRef CAS
; (b) D. A. C. Stuerga and P. Gaillard, J. Microwave Power Electromagn. Energy, 1996, 31, 101
.
-
(a) N. Kuhnert, Angew. Chem., Int. Ed., 2002, 41, 1863 CrossRef CAS
; (b) Ch. Strauss, Angew. Chem., Int. Ed., 2002, 41, 3589 CrossRef CAS
.
- M. Larhed and A. Hallberg, Drug Discovery Today, 2001, 5, 406 CrossRef CAS
.
- A. Matlack, Green Chem., 2003, 5, G7 RSC
.
- D. Adam, Nature, 2003, 421, 571 CrossRef CAS
.
- Search in SciFinder Scholar: “Microwave synthesis, not plasma, not discharge, not spectroscopy”, Dec. 2003.
- R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett., 1986, 27, 279 CrossRef CAS
.
- R. J. Giguere, T. L. Bray, S. M. Duncan and G. Majetich, Tetrahedron Lett., 1986, 27, 4945 CrossRef CAS
.
- X. Yu and X. Huang, Synlett, 2002, 1895 CAS
.
- S. Balalaie, M. M. Hashemi and M. Akhbari, Tetrahedron Lett., 2003, 44, 1709 CrossRef CAS
.
- B. Kumar, B. Kaur, J. Kaur, A. Parmar and H. Kumar, Indian J. Chem., Sect. B, 2002, 41, 1526
.
- http://www.pueschner.com/dt/basics .
- R. S. Varma, R. Dahiya and S. Kumar, Tetrahedron Lett., 1997, 38, 2039 CrossRef CAS
.
-
(a) L. Perreux and A. Loupy, Tetrahedron, 2001, 57, 9199 CrossRef CAS
; (b) L. Perreux, A. Loupy and F. Volatron, Tetrahedron, 2002, 59, 2155 CrossRef
; (c) L. Perreux, A. Loupy and M. Delmotte, Tetrahedron, 2003, 59, 2185 CrossRef CAS
.
- Z. Zhang, L. Zhou, M. Zhang, H. Wu and Z. Chen, Synth. Commun., 2001, 31, 2435 CrossRef CAS
.
-
(a) R. Carta and L. Loddo, Ind. Eng. Chem. Res., 2002, 41, 5912 CrossRef CAS
; (b) D. Stuerga, K. Gonon and M. Lallemant, Tetrahedron, 1993, 49, 6224 CrossRef CAS
.
- S.-T. Chen, P.-H. Tseng, H.-M. Yu, C.-Y. Wu, K.-F. Hsiao, S.-H. Wu and K.-T. Wang, J. Chin. Chem. Soc., 1997, 44, 169 CAS
.
- R. Dagani, Chem. Eng. News, 1997, 75, 26
.
- D. R. Baghurst and D. M. P. Mingos, J. Chem. Soc., Chem. Commun., 1992, 674 RSC
.
- A. Stadler and C. O. Kappe, Eur. J. Org. Chem., 2001, 919 CrossRef CAS
.
-
Ch. Wohlfarth, “Temperature Dependence of the Permittivity (Dielectric Constant) of Liquids”, in CRC Handbook of Chemistry and Physics, ed. D. R. Lide, 76th edn., CRC Press, Boca Raton, Ann Arbor, London, Tokyo, 1992, Sect. 6, p. 193 Search PubMed
.
- U. Kaatze, Radiat. Phys. Chem., 1995, 45, 539 CrossRef CAS
.
- B. R. Pujari, B. Barik and B. Becera, Phys. Chem. Liq., 1998, 36, 105 Search PubMed
.
- W. Lautenschläger and T. Schweizer, Laborpraxis, 1990, 5, 376 Search PubMed
.
- M. C. Bagley, R. Lunn and X. Xiong, Tetrahedron Lett., 2002, 43, 8331 CrossRef CAS
.
- U. Müller, M. Nüchter and B. Ondruschka, Bioforum, 2000, 23, 406 Search PubMed
(CAN 134: 193008)..
- A. Stadler, S. Pichler, G. Horeis and C. O. Kappe, Tetrahedron, 2002, 58, 3177 CrossRef CAS
.
- N. E. Leadbeater and M. Marco, J. Org. Chem., 2003, 68, 888 CrossRef CAS
.
- C. O. Kappe, D. Kumar and R. S. Varma, Synthesis, 1999, 1799 CrossRef CAS
.
- M. Mihara, Y. Ishino, S. Minakata and M. Komatsu, Synlett, 2002, 1526 CAS
.
- S. Paul, P. Nanda, R. Gupta and A. Loupy, Tetrahedron Lett., 2002, 43, 4261 CrossRef CAS
.
- Method for measurement serviceability of microwave devices for domestic use and similar purposes, European standard SN 60706, 1995.
- C. A. Vriezinga, J. Appl. Phys., 1999, 85, 3774 CrossRef CAS
.
-
M. Nüchter and B. Ondruschka, unpublished results, Jena 2000–2003
.
- H. Koshima and K. Kitsunai, Heterocycles, 2002, 57, 1299 CAS
.
-
(a)
http://www.cemsynthesis.com, for detailed description of PowerMAX concept see;
(b) J. D. Ferguson, Mol. Diversity, 2003, 7, 281 Search PubMed
; (c) J. J. Chen and S. V. Deshpande, Tetrahedron Lett., 2003, 44, 8873 CrossRef CAS
; (d) U. Sengutta and H.-P. Meier, GIT Labor-Fachz., 2002, 1038 Search PubMed
(CAN 137:202233)..
-
M. Nüchter, B. Ondruschka, W. Bonrath, D. Weiß and R. Beckert, unpublished results, Jena, 2003
.
-
(a) K. Jinno, Anal. Bioanal. Chem., 2002, 373, 1 CrossRef CAS
; (b) C. W. Huie, Anal. Bioanal. Chem., 2002, 373, 23 CrossRef CAS
; (c) L. M. Assis, J. S. S. Pinto and F. M. Lancas, J. Microcolumn Sep., 2000, 12, 292 CrossRef CAS
.
- M. Letellier and H. Budzinski, Analusis, 1999, 27, 259 CrossRef CAS
.
-
(a) C. Struppe, M. Nüchter and B. Ondruschka, Chem. Tech. (Leipzig), 1999, 51, 127 Search PubMed
(CAN 130: 352026).; (b) M. Nüchter, B. Ondruschka, St. Knecht, U. Nüchter and K. Fischer, Chem. Tech. (Leipzig), 2000, 52, 133 Search PubMed
(CAN 134: 197535)..
- W. Lautenschläger, B. Ondruschka and M. Nüchter, Ger. Offen. DE, 2000, 100 246 31.
- http://www.oc-praktikum.de. This is an internet project of eight German research groups for bettering the education of chemistry students with modern aspects and detailed description of several experiments, also with alternative energy entries, facts about toxicology and ecotoxicology.
-
G. D. Beal, Org. Synth., Coll. Vol. I, John Wiley & Sons Inc., New York, 1932, 538 Search PubMed
.
-
(a)
F. Fringuelli and A. Taticchi, Dienes in the Diels–Alder Reaction, John Wiley & Sons Inc., New York, 1990 Search PubMed
; (b) H. Wollweber, Diels–Alder Reaction, Thieme, Stuttgart, Germany, 1972 Search PubMed
.
- P. Yates and P. Eaton, J. Am. Chem. Soc., 1960, 82, 4436 CrossRef CAS
.
- W. E. Bachmann and L. B. Scott, J. Am. Chem. Soc., 1948, 70, 1458 CrossRef CAS
.
-
(a) R. Schlögl, Angew. Chem., Int. Ed., 1998, 37, 2333 CrossRef CAS
; (b) R. P. Hertzberg and A. J. Pope, Curr. Opin. Chem. Biol., 2000, 4, 445 CrossRef CAS
; (c) E. H. Ohlstein, R. R. Ruffolo Jr. and J. D. Elliott, Annu. Rev. Pharmacol. Toxicol., 2000, 40, 177 CrossRef CAS
.
- I. A. Cotterill, A. Y. Usyatinsky, J. M. Arnold, D. S. Clark, J. S. Dorick, P. C. Michels and Y. L. Khmelnitsky, Tetrahedron Lett., 1998, 39, 1117 CrossRef
.
- A. Lew, P. O. Krutzik, M. E. Hart and A. R. Chamberlin, J. Comb. Chem., 2002, 4, 95 CrossRef CAS
.
-
C. Kappe and A. Stadler, Microwaves in Organic Synthesis, ed. A. Loupy, Wiley-VCH, Weinheim, 2002, ch. 12, p. 405 Search PubMed
.
-
(a) M. Nüchter, B. Ondruschka, A. Tied, W. Lautenschläger and K. J. Borowski, American Genomic/Proteomic Technology, Sept./Oct. 2001, 34 Search PubMed
(CAN 138: 72785).; (b) J. Freitag, M. Nüchter and B. Ondruschka, Green Chem., 2003, 5, 291 RSC
; (c) M. Nüchter and B. Ondruschka, Mol. Diversity, 2003, 7, 253 Search PubMed
.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS
.
- Y.-S. Hon, T.-R. Hsu, C.-Y. Chen, Y.-H. Lin, F.-J. Chang, C.-H. Hsieh and P.-H. Szu, Tetrahedron, 2003, 59, 1509 CrossRef CAS
.
- G. R. Brown, A. J. Foubister, C. A. Roberts, S. L. Wells and R. Wood, Tetrahedron Lett., 2001, 42, 3917 CrossRef CAS
.
- Based on the fundamentals of wave optics, the construction characteristics of the microwave apparatus, and on our own research and development work with the various systems, the authors are of the opinion that the DISCOVER™- and EMRYS™-series microwave systems do not function exclusively with a monomode character.
-
B. Ondruschka and M. Nüchter, “Recent Applications of Microwave Power for Applied Organic Chemistry”, in Advances in Microwave and Radio Frequency Processing, ed. M. Willert-Porada, Springer Verlag, Berlin – Heidelberg, 2003, 387 Search PubMed
.
-
(a) R. Bierbaum, M. Nüchter and B. Ondruschka, Chem. Ing. Tech., 2003, 75, 1042 CrossRef CAS
; (b) R. Bierbaum, M. Nüchter, B. Ondruschka, Microwave-Assisted Reactions in a Miniplant Setup with On-Line HPLC Analysis and Automated Process Control – Preliminary Results, Book of Abstracts, 9thInternational Conference on Microwave and High Frequency Heating, Loughborough, UK, 2003 Search PubMed
.
-
(a) G. Pipus, I. Plazl and T. Koloini, Ind. Eng. Chem. Res., 2002, 41, 1129 CrossRef CAS
; (b) X. Liao, G. S. V. Raghavan and V. A. Yaylayan, Tetrahedron Lett., 2002, 43, 45 CrossRef CAS
.
-
(a) E. Esveld, F. Chemat and J. v. Haveren, Chem. Eng. Technol., 2000, 23, 279 CrossRef CAS
; (b) E. Esveld, F. Chemat and J. v. Haveren, Chem. Eng. Technol., 2000, 23, 429 CrossRef CAS
.
- Authors team, “Organikum”, 20th edn., Wiley-VCH, Weinheim, New York, 1999, 444
.
-
(a) M. F. Malone and M. F. Doherty, Ind. Eng. Chem. Res., 2000, 39, 3953 CrossRef CAS
; (b) S. Steinigeweg and J. Gmehling, Ind. Eng. Chem. Res., 2002, 41, 5483 CrossRef CAS
.
- P. Moritz, S. Blagov and H. Hasse, Chem. Ing. Tech., 2003, 74, 1207 CrossRef
.
-
H. Drost, “Plasmachemie”
Akademie-Verlag, Berlin, 1978, 69
.
-
W. Bonrath, F. Cirillo, US 2000, 6444098
.
- R. A. Sheldon, J. Mol. Catal. A: Chem., 1996, 107, 75 CrossRef CAS
.
- W. Bonrath, Ultrason. Sonochem., 2003, 10, 55 CrossRef CAS
; W. Bonrath, Ultrasonics Sonochem., 2004, 11, 1 Search PubMed
.
-
J. A. Kerr, “Strengths of Chemical Bonds” in CRC Handbook of Chemistry and Physics, ed. D. R. Lide, 76th edn., CRC Press, Boca Raton, Ann Arbor, London, Tokyo, 1992, Sect. 9, p. 51 Search PubMed
.
-
P. W. Atkins
Physical Chemistry
Oxford University Press
Oxford, 1990, p. 938 Search PubMed
.
- D. A. C. Stuerga and P. Gaillard, J. Microwave Power Electromagn. Energy, 1996, 31, 87
.
- R. S. Varma and V. V. Namboodiri, Chem. Commun., 2001, 643 RSC
.
- J.-S. Schanche, Mol. Diversity, 2003, 7, 293 Search PubMed
.
- L. Favretto, Mol. Diversity, 2003, 7, 287 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2004 |
