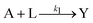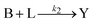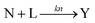A study of the elemental leachability and retention capability of compost
Qi Jun Song and Gillian M Greenway*
Department of Chemistry, University of Hull, Cottingham Road, Hull, UK HU6 7RX. E-mail: g.m.greenway@hull.ac.uk
First published on 26th November 2003
Abstract
In this work a comparison is made between the different approaches that can be taken to evaluate the mobility of elements in compost. The practical consequences of the results obtained are also discussed in terms of methods for cleaning up compost and using compost in environmental remediation. The mobility of potentially toxic elements in compost is evaluated by leaching with four selected eluents, i.e. diluted sulfuric acid, oxalate, citrate and EDTA. In contrast to the chelating agents, diluted sulfuric acid was found to generally have a low leaching capability for removal of heavy metals from compost. This implies that the risk of heavy metal leaching caused by natural rainfall is likely to be low. The results obtained in the leaching experiment were compared with previous results obtained from sequential fractionation. This comparison confirmed that both methods gave similar results for predicting the lability of elements in compost. A non-linear regression analysis of the leaching curves was also conducted. The leaching curves for elements with high lability could be fitted with a two components model. The labile components identified by the kinetic model are approximately in accordance with the fractions obtained from the first step of the sequential extraction method. The kinetic speciation method is shown to be a relatively rapid and simple procedure for compost which gives more information about element lability than simple leaching experiments. The leaching reagents used in this work were not effective enough to be used for cleaning up compost with a high metal content. Compost was however shown to have a high affinity for heavy metals, with the order of affinity of metal for the compost being very similar to that seen for humic acid. Compost may therefore prove to be a good remediation material for metal contaminated waste.
Introduction
Composting has become increasingly important for solid waste disposal, because it provides an efficient and environment friendly method to reduce the volume of waste. The composting process also can detoxify harmful organic substances and pathogens, and provides a material of agricultural importance.1,2 Recently however, there have been concerns over its safe application in the ecosystem due to the introduction of industrial or municipal solid waste (MSW) to composting waste, which may increase its heavy metal content. When measuring the element content in compost, the quantification of the chemical forms is essential for estimating the mobility and bioavailability of the elements in the composts. Various sequential extraction protocols had been proposed and utilized to allow fractionation of the elements into operationally defined geochemical phases for the analysis of soils and sediments.3,4 More recently, these procedures have been applied to compost.5–8 It has also been shown that this time-consuming process can be speeded up substantially if an ultrasonically accelerated version of sequential extraction is utilized.9 Using this rapid method it was found that elements such as Zn, Mn and As had a high potential mobility in compost, whereas other elements such as Pb, Cu, Fe, and Cr exist mainly in immobilized forms. This approach to measuring elemental mobility has however been criticized for various reasons. The first step of the procedure often involves extraction with 0.1 mol l−1 acetic acid which is a relatively strong extractant and as it removes all the water-, acid soluble and exchangeable forms together it cannot give information about the current mobility as opposed to the potential mobility of the sample.10 Another major criticism is that the extraction schemes are performed under pseudo-equilibrium conditions and therefore the information on the availability of the element is only based on thermodynamic considerations.11The leaching property of a waste material is an important criterion for the management of waste. Many leaching tests are developed as standard methods for evaluating the potential impacts of waste material on the environment.12 For example, the Environmental Protection Agency of the United States have developed a toxicity characterization leaching procedure (TCLP) which is frequently used to evaluate the mobility of both organic and inorganic components present in contaminated soil and other waste materials.13,14 Leaching experiments can also be used as a complementary tool to assess the mobility of heavy metals and metalloids in compost. With leaching procedures, the experiments can be carried out so as to mimic various natural scenarios such as rainfall giving more realistic information about the element mobility.10,15
Leaching experiments are also useful for the evaluation of possible clean-up techniques for compost as changes in environment conditions may lead to the risk of heavy metals being released to the environment.15 Compost may therefore have to be cleaned up before application to land or even before the composting process has been carried out.7 One way to clean up the compost is to use a flushing technique with leaching reagents. This approach has been more frequently investigated for cleaning heavy metal contaminated soil.16–18
Further kinetic information can be obtained from leaching experiments by recording the amount of leached element versus leaching time for a given reagent. Several authors consider this approach is more likely to give a more real distribution of elemental species as found in the natural environment.19,20 By applying a non-linear regression model, the leached species can be categorized into two types, i.e. those which are easily extracted (labile species) and those which are extracted more slowly (non-labile species).20,21 There is no report of such kinetic speciation methods having been applied to compost samples.
Finally a retention experiment was carried out to assess the ability of compost to adsorb microelements in water. The aim of this experiment was to see if the application of compost in remediation technologies could be expanded to cleaning up water contaminated with heavy metals.
Experimental
Reagents, instruments and compost materials
A solution of 0.005 mol l−1 H2SO4 was diluted directly from a super purity grade reagent purchased from Romil Ltd., Cambridge, UK. Other leaching reagents including 0.05 mol l−1 ammonium oxalate, 0.05 mol l−1 ammonium citrate, and 0.05 mol l−1 EDTA, were prepared by dissolving an appropriate amount of the corresponding chemicals (BDH Laboratory Supplies, Poole, UK) in water and adjusting the pH to 5.5 with a pH meter (Fisherbrand Hydrus 500 pH Meter, Fisher Scientific UK Ltd. Loughborough, UK). All the standard element solutions were prepared by dilution from a 1000 ± 3 µg ml−1 standard solution (Peak Performance, Qmx Laboratories limited, UK). High purity de-ionised water (18 MΩ cm resistance) was obtained from an Elgstat UHQ PS system (Elga, High Wycombe, UK). The element analysis was performed with a quadrupole inductively coupled plasma mass spectrometer (ICP-MS), from ThermoElemental, Winsford, Cheshire, UK.The compost samples were taken from mature compost, consisting of wood (from demolition sites), straw and vegetable waste. The details of the site description and sampling procedures have been reported previously.9
Leaching experiment
The leaching experiment was carried out in packed columns in which 2.5 g samples of ground compost (<1 mm) were dry packed into standard 50 ml glass burettes giving a sample length of about 9 cm. Glass wool was placed at the bottom of the columns to stop any loss of solid particles. A 2 cm plug of glass wool was packed on top of the column to prevent the eluent from disturbing the column of compost. Eluent was added to the top of each packed column, and was allowed to pass through the column at a rate of 0.25 ml min−1. As long as the liquid head on the column was maintained, this flow rate remained fairly constant over the whole process. The leachate was collected in a polyethylene sample tube, with fractions collected more frequently at the beginning of the experiments where rapid changes in concentration were occurring (Fig 2). The pH of the leachate from the eluent of sulfuric acid was also monitored. The column leaching was completed in 12 h and duplicate experiments were carried out for all the eluents.The non-linear regression study of the leaching data was carried out using SigmaPlot8.0, a software package produced by SPSS Ltd.
Retention experiment
The columns of compost that had been leached with EDTA were further conditioned with 0.005 mol l−1 H2SO4 and Elga UHQ water to remove all the chemical residues. Then 5 µg ml−1 standard solutions of the selected elements was continuously added on the top of each column and the solution was allowed to pass through the column at a flow rate of 0.25 ml min−1. The eluted solutions were collected in 10 ml aliquots and all the solutions were stored at 4 °C in a cold room before analyzing by ICP-MS.Results and discussion
Leaching properties of elements in compost
A mature compost sample taken from day 128 of the composting procedure was selected for the leaching experiment. For easy comparison, the results obtained previously for sequential extraction from this compost are presented in Fig. 1 for the elements used in this study.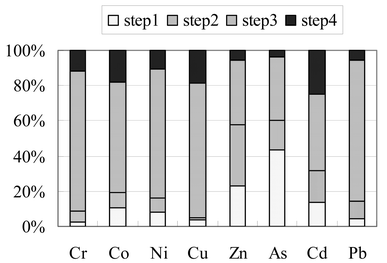 | ||
| Fig. 1 The elemental partitioning results of the mature compost (day 128) used in the leaching experiment.9 Step1 – the easily extractable fraction; Step 2 – reducible fraction; Step 3 – bound with organic materials; Step 4 – residues. | ||
In Fig. 1 Step 1 is the fraction extracted by 0.11 mol l−1 acetic acid which represents the most labile components in the compost. Step 2 is extracted with 0.1 mol l−1 hydroxylamine hydrochloride and represents the reducible fractions or the fractions associated with iron–manganese oxides. Step 3 is the combined results for an extraction with 0.1 mol l−1 sodium pyrophosphate followed by an extraction with 1 mol l−1 ammonium acetate after digestion with hydrogen peroxide. Therefore Step 3 in Fig. 1 represents the overall fractions that are bound with organic materials. The results for Step 4 are obtained from a nitric acid digestion, which represents the residue part of the compost. This type of sequential extraction procedure has been developed for soils and sediments which have different characteristics to compost. This paper describes a range of leaching and sorption experiments performed on compost to more comprehensively evaluate the characteristics of heavy metals and metalloids in composts.
The leaching reagents used in the study are given in Table 1. Sulfuric acid was chosen to mimic the acid rain scenario. The amount of acid added was approximately equivalent to two years of rainfall in the UK percolating through a 9 cm thickness of compost layer. The acidity was deliberately chosen to be stronger than any realistic acid rain to maximise the potential risk of heavy metal release. EDTA was also selected as a leaching agent because it is known to be a strong chelating agent and has been frequently utilized to clean contaminated soil and in kinetic studies.11,18 Citrate and oxalate ligands also have the ability to complex with various heavy metals, but they are natural products and biodegradable which makes them superior to EDTA for application as environmental remediation agents.18 Oxalate is also known to be a strong reducing agent, which means it is more likely to release the elements trapped in iron–manganese oxides. A concentration of 0.05 mol l−1 and pH 5.5 was chosen for all the chelating agents so that their leaching ability could be compared without being significantly affected by the differences in ionic strengths and acidity. These conditions were selected because they had been shown to be optimum for soil leaching experiments by a number of previous workers.11,20,21 These levels were also chosen because any higher acidity of sulfuric acid would compromise its complexing ability and a lower acidity (high pH) may cause significant loss of humic substances. The changes in pH of the leachate was monitored from the sulfuric acid leaching columns and this indicated that the compost had a high ion exchange capacity. It was found that the pH remained neutral until 80 ml of 0.005 mol l−1 H2SO4 had passed through the column. This occurred because protons in the acid solution exchanged with macroelements such as Na+, K+ and Ca2+ that were loosely bound to the compost material. Based on the air-dried sample weight, the cation exchange capability (CEC) of the compost was calculated to be 32 cmol kg−1, which is slightly higher than the previous values reported for the MSW composts.5
| 1 | 0.005 mol l−1 H2SO4 |
| 2 | 0.05 mol l−1 ammonium oxalate, pH 5.5 |
| 3 | 0.05 mol l−1 ammonium citrate, pH 5.5 |
| 4 | 0.05 mol l−1 EDTA, pH 5.5 |
The leaching data, which were expressed as the cumulative percentage of elements removed from the compost versus the time taken for leaching, is presented in Fig. 2.
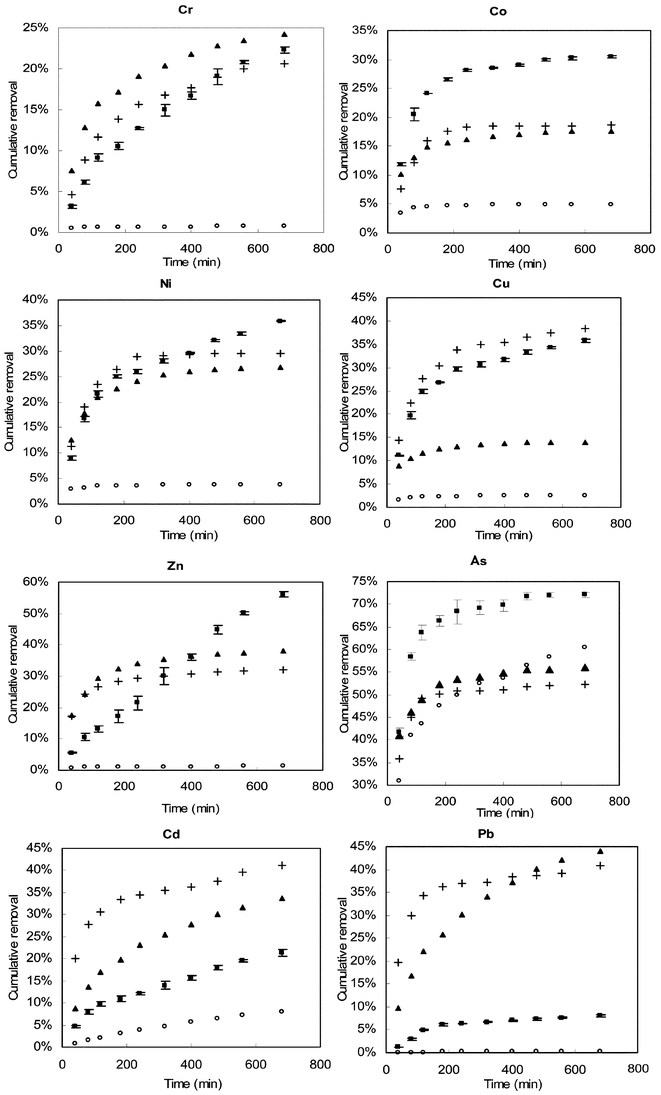 | ||
| Fig. 2 Results of column leaching experiment; ■ oxalate, ▲ citrate, + EDTA, ○ sulfuric acid. | ||
For clarification, the error bars of the duplicate leachings are only given for one of the experiments, which had the worst reproducibility, leaching with oxalate. These results however show that the general trends discussed are greater than the errors in the data. The results obtained for each element are discussed as follows:
It is evident from the above results, that the removal of heavy metals from compost may be more difficult than from soil, as with similar leaching procedures the removal rate in soil were generally much higher.18
From the previous discussions it can be concluded that leaching experiments can provide useful information about element mobility and also allow the assessment of possible cleanup procedures for contaminated composts. Generally sulfuric acid was shown to remove very little heavy metal from the compost, which means that the release of heavy metal from compost by acid rain is not a serious risk. In the cases of Zn, As and Cd, however, the amount leached by diluted sulfuric acid was higher, which means compost with high levels of these elements could pose a higher risk of release to the environment. In terms of heavy metal removal, the results show that the process of leaching composts with chelating agents is generally not as efficient as for soil. This is due to the fact that most heavy metals are strongly complexed with humic substances in compost.9,24 In many cases oxalate has shown its extra reducing capability by releasing the metals trapped in iron and manganese oxides. EDTA was the most efficient leaching reagent for the removal of Zn, Cd and Pb.
Kinetic speciation of the trace elements in composts
Kinetic approaches to chemical speciation have been applied to the assessment of heavy metal mobility in soils and sediments and have the advantage of being simple and rapid.20,21 By applying a mathematical model to the data from the leaching experiments , it is possible to differentiate the chemical species based on their different leaching rates. The extraction process can be described by the following reactions:Where A, B, …, and N represent different components containing the analyte of interest and their initial amounts are a, b,..., n respectively. L represents the leaching agent which exists in a large excess. Y is the product formed and its concentration y is monitored by an analytical method. k1, k2, – kn are the rate constants of the corresponding reactions. If a large excess of L is maintained in the leaching process, then the leaching reactions can be considered as pseudo first-order reactions. The time-dependent concentration profile of the product Y is described by the following expression,25,26
| y = a(1 − e−k1t) + b(1 − e−k2t) + ··· + n(1 − e−knt) | (1) |
Of the leaching agents used in this work it has been shown that dilute sulfuric acid only released very labile species and therefore further discrimination may not be necessary. For oxalate, the mechanism of the leaching process is theoretically too complicated for the model, as complexation, reduction and precipitation reactions may all be simultaneously involved in the leaching process. It was therefore decided to only apply this non-linear model to the data obtained from the EDTA and citrate leaching experiments. It was found that a one-component model could be readily obtained for all the leaching curves, even though the actual fittings were not very satisfactory by visual inspection of the fitted curves and the experimental data. When the two-component model was applied to the EDTA leaching data for Cr, Co, Ni, Cu, Cd, and Pb, it simply did not converge or the parameters exceeded the maximum number of iterations. Similar results were obtained for the citrate leaching data for Cr, Cu, Cd, and Pb, which was probably due to the fact that the leaching rate of the labile component were close to that of non-labile component (k1/k2 ≪ 10) or the fraction of labile component was too small to be identified based on the leaching data. In the cases of Zn and As, however, the two-component model did give excellent fit for both of the EDTA and citrate leaching curves. Co and Ni leaching curves could also be better fitted to a two-component model when citrate was used as an eluent. The fitted parameters and statistics for the two-component models are given in Table 2 and an example of the fitted curves obtained from different models is given in Fig. 3.
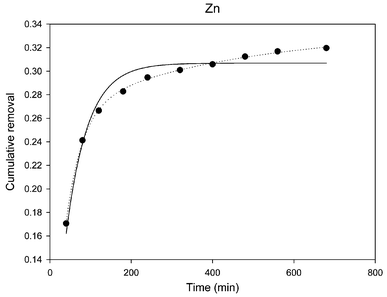 | ||
| Fig. 3 Non-linear regression curve of Zn obtained from EDTA leaching data; ● experimental data, — one-component model, ··· two-component model. | ||
| Element | Fitted parameters | Rsqr. | F | P |
|---|---|---|---|---|
| Zn (EDTA) | a = 0.2557 ± 0.0096; k1 =0.0250 ± 0.0013 | 0.9956 | 1194 | 0.0001 |
| b = 0.0846 ± 0.0082; k2 =0.0023 ± 0.0010 | ||||
| As (EDTA) | a = 0.4642 ± 0.0175; k1 =0.0335 ± 0.0022 | 0.9826 | 300.3 | 0.0001 |
| b = 0.0648 ± 0.0097; k2 =0.0033 ± 0.0022 | ||||
| Co (Citrate) | a = 0.1193 ± 0.0063; k1 =0.0345 ± 0.0026 | 0.9957 | 1247 | 0.0001 |
| b = 0.0583 ± 0.0054; k2 =0.0054 ± 0.0008 | ||||
| Ni (Citrate) | a = 0.1465 ± 0.0200; k1 =0.0315 ± 0.0061 | 0.9870 | 404.4 | 0.0001 |
| b = 0.1289 ± 0.0153; k2 =0.0045 ± 0.0011 | ||||
| Zn (Citrate) | a = 0.3075 ± 0.0218; k1 =0.0160 ± 0.0012 | 0.9961 | 1353 | 0.0001 |
| b = 0.1339 ± 0.0790; k2 =0.0012 ± 0.0016 | ||||
| As (Citrate) | a = 0.4033 ± 0.0144; k1 =0.0652 ± 0.0088 | 0.9856 | 364.9 | 0.0001 |
| b = 0.1608 ± 0.0116; k2 =0.0054 ± 0.0008 |
The rate constant can be considered as a measure of the lability, therefore based on the data in Table 2 it may be sensible to say that As is more labile than Zn. For the leaching experiment with EDTA the ratios of k1/k2 are 10.9 and 10.2 respectively for Zn and As which means that the leaching rate of the labile component is significantly higher than that of non-labile component. The results also indicate that 25.6% and 46.4% of the Zn and As respectively leached by EDTA are from labile components. These figures are surprisingly close to the values for Zn and As that were extracted in the first step of the results obtained from the previous sequential extraction method (see Fig. 1). On this occasion, these two operationally defined speciation methods agreed very well in defining the labile species, though by a very different approach.
From the two-component model of citrate leaching, the labile components are 11.9%, 14.6%, 30.8%, and 40.3% respectively for Co, Ni, Zn, and As (Table 2). These figures again approximately agreed with the first step fractions obtained by the sequential extraction method (see Fig. 1). By comparing the k1 values, the order of the lability of these four elements can be given: As > Co > Ni > Zn. The differences in leaching rate of the two components (k1/k2) are appreciable, being 6.4, 7.0, 13.3, and 12.1 for Co, Ni, Zn, and As respectively.
For these experiments, with citrate as the leaching agent two kinetically distinguishable components could be identified for four elements whereas only two could be seen for EDTA. This is probably due to the fact that EDTA is a much stronger chelating agent than citrate (for example, log KZn–EDTA = 16.5, log KZn–citrate = 11.4). Consequently it is less likely that EDTA can differentiate between the leaching rates, as Co and Ni have a relatively low k1/k2 ratios compared to As and Zn. From this point of view, strong chelators are not always appropriate for the purpose of identifying kinetic differences.
The amounts of components classified as labile by citrate was also slightly different to that obtained from EDTA. This should be expected as lability is a loosely defined concept and the lability of a species is highly dependant on the reagents to which it refers.
Retention of trace elements by compost
It is useful to know if after a leaching experiment the compost sample can re-adsorb the trace elements and the preference of elemental adsorption. For this purpose, a solution containing 5 µg ml−1 of each element in the study was added to the conditioned columns. The eluted solutions were then analysed. The concentrations of elements in each aliquot solution collected were plotted against the liquid to solid ratio as shown in Fig. 4. These results show, as would be expected, that the compost exhibits different affinities for different elements. As has the least affinity for compost and therefore was the first element to breakthrough, with the eluent concentration leveling off around the concentration initially added. Co, Zn, Cd, and Ni were consecutively leached out. For these elements it was interesting to note that once these elements start to leach, the concentrations of the collected solutions were greater than that of the initial concentration of added solution. Presumably this is because the previously adsorbed elements were replaced by subsequent elements which had stronger affinity to compost. Pb, Cr and Cu demonstrated the strongest affinity to compost, and even at the end of the experiment their concentrations were still below the initial levels, that means that the compost is still not saturated. From the mass balance, it can be calculated that 1 g of compost had adsorbed more than 300 µg of Pb in the presence of other coexistent elements.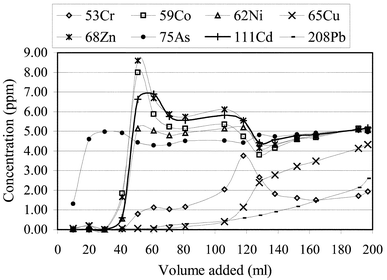 | ||
| Fig. 4 Retention experiment result. The eluent contains 5 ppm (µg ml−1) of Cr, Co, Ni, Cu, Zn, As, Cd and Pb with the pH of the solution being 4.0. | ||
The overall order of the affinity of compost to the elements in the study would be: Pb> Cu > Cr ≫ Ni ≥ Cd ≥ Zn ≥ Co ≫ As. This sequence could be explained by the affinity of humic acids for these elements24,27 and tends to confirm that humic acids play a major role in the sorption of heavy metals by compost.
In conclusion this compost has high affinity to Pb, Cu and Cr, intermediate affinity to Ni, Cd, Co and Zn, and least affinity to As. The results imply that compost could be used as an absorbent to clean up heavy metals in contaminated water.
It is worth noting, however, that solutions in the retention experiment were prepared directly from standard solution, their chemical forms are much simpler than the species encountered in most real contamination cases. It would therefore be worth carrying out further investigations to see if the compost could be applied to real samples, for example, landfill leachate.
Conclusions
In this paper the microelement leaching property in compost was investigated and the results obtained were compared with those obtained by sequential extraction methods. It was shown that dilute sulfuric acid was ineffective at leaching the elements in the study with the exception of arsenic. In terms of heavy metal removal, none of the eluents investigated were very efficient, which reflects the fact that components of the compost have a strong affinity to heavy metals. Oxalate was shown to have an additional leaching capability due to its reducing properties, which meant it was able to release metals trapped in iron–manganese oxides. In terms of identifying the mobility of elements in compost, the results from the leaching experiments generally agreed with those previously obtained by the sequential extraction method. This confirms that both methods provide a useful tool for finding out the labile forms of an element in compost, although the sequential method will give further information about complexation to different solid phases.The kinetic speciation method was then applied to data obtained from the leaching experiments and the results obtained were promising. The amount of labile component identified by this method was also found to be in good agreement with the labile fractions extracted by the sequential extraction method.
In conclusion, the kinetic speciation method is a relatively rapid and simple procedure which gives more information about element lability than simple leaching experiments. In future it would be useful to apply this method to a sample with well-characterised geochemical phases to further prove its preciseness.
Sorption experiments confirmed that the compost has a high affinity to heavy metals and very low affinity to arsenic. The order of affinity that the various heavy metals had for compost was the same as previously reported for humic acid, i.e.: Pb > Cr > Cu > Ni ≥ Cd ≥ Zn ≥ Co, suggesting that humic acids are the main sorption materials in the compost. To fully exploit the feasibility of using compost as a remediation material, sorption experiments with real contaminated samples will need to be conducted in the future.
Acknowledgements
Authors would like to thank Shanks First for the financial support of this project and Dr. A. Walmsley and Dr. J. Adams at the University of Hull for their useful advice on the computer modeling methods.References
- A. Gomez, Trends Anal. Chem., 1998, 17, 310 CrossRef CAS.
- A. M. Fogarty and O. H. Tuovinen, Microbiol. Rev., 1991, 55, 225 Search PubMed.
- A. Tessier, P. G. C. Campbell and M. Bisson, Anal. Chem., 1979, 51, 844 CrossRef CAS.
- A. M. Ure, P. Quevauviller, H. Muntau and B. Griepink, Int. J. Environ. Anal. Chem., 1993, 51, 135 CrossRef CAS.
- X-T. He, T. J. Logen and S. J. Traina, J. Environ. Qual., 1995, 24, 543 CAS.
- J. Chwastowska and K. Skalmowski, Int. J. Environ. Anal. Chem., 1997, 68, 13 CAS.
- T. Korolewicz, M. Turek, J. Ciba and J. Cebula, Environ. Sci. Technol., 2001, 35, 810 CrossRef CAS.
- G. M. Greenway and Q. Song, J. Environ. Monit., 2002, 4, 300 RSC.
- G. M. Greenway and Q. Song, J. Environ. Monit., 2002, 4, 950 RSC.
- P. Anderson, C. M. Davidson, A. L. Duncan, D. Littlejohn, A. M. Ure and L. M. Garden, J. Environ. Monit., 2000, 2, 234 RSC.
- D. Fangueiro, A. Bermond, E. Santos, H. Carapuca and A. Duarte, Anal. Chim. Acta, 2002, 459, 245 CrossRef CAS.
- H. A. Van der Sloot, Trends Anal. Chem., 1998, 17, 298 CrossRef CAS.
- X. B. Chen, J. V. Wright, J. L. Conca and L. M. Peurrung, Water Air Soil Pollut., 1997, 98, 57 CrossRef CAS.
- A. Majid and S. Argue, Miner. Eng., 2001, 14, 1513 CrossRef CAS.
- L. Gove, C. M. Cooke, F. A. Nicholson and A. J. Beck, Bioresour. Technol., 2001, 78, 171 CrossRef CAS.
- C. G. Rampley and K. L. Ogden, Environ. Sci. Technol., 1998, 32, 987 CrossRef CAS.
- M. A. Yukselen and B. Alpaslan, J. Hazard. Mater., 2001, B87, 289 CrossRef.
- S. A. Wasay, S. Barrington and S. Tokunaga, Water Air Soil Pollut., 2001, 127, 301 CrossRef CAS.
- Z. B. Li and L. M. Shuman, Soil Sci., 1996, 161, 226 CrossRef CAS.
- J. Yu and D. Klarup, Water Air Soil Pollut., 1994, 75, 205 CAS.
- A. Bermond, I. Yousfi and J-P. Ghestem, Analyst, 1998, 123, 785 RSC.
- J. C. Chang, Solubility Product Constants, in CRC Handbook of Chemistry and Physics, 72nd edn., D. R. Lide, 1991–1992.
- The Composting Association Standards for Composting, Briefing Note, The Composting Association (UK), May 2000.
- H. Kerndorff and M. Schnitzer, Geochim. Cosmochim. Acta, 1980, 44, 1701 CrossRef CAS.
- M. K. S. Mak and C. H. Langford, Inorg. Chim. Acta, 1983, 70, 237 CrossRef CAS.
- H. L. Pardue, Anal. Chim. Acta, 1989, 216, 69 CrossRef CAS.
- L. Madronová, J. Kozler, J. Čežíková, J. Novák and P. Janoš, React. Funct. Polym., 2001, 47, 119 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |