An advanced biological scanning electrochemical microscope (Bio-SECM) for studying individual living cells
Luciana
Pitta Bauermann
,
Wolfgang
Schuhmann
and
Albert
Schulte
*
Anal. Chem. -Elektroanalytik & Sensorik, Ruhr-Universität Bochum, Bochum, D-44780, Germany. E-mail: albert.schulte@ruhr-uni-bochum.de; Fax: +49 234 32 14683; Tel: +49 234 32 26202
First published on 24th June 2004
Abstract
An inverted optical microscope was customized to incorporate a constant-distance mode scanning electrochemical microscope (SECM). The system worked well with an optical, shearforce-based feedback mechanism for maintaining a constant tip-to-sample separation throughout scanning. The highly accurate distance control of the established Bio-SECM allowed novel, flexible carbon-fibre microelectrodes with appropriate vibration characteristics and significantly reduced tip diameters to be used as vibrationable SECM tips for topographical and electrochemical measurements on soft biological samples such as adherently growing fibroblasts or adrenal chromaffin cells. Visual aid offered by the optical microscope helped identifying suitable cells and supported manual prepositioning of an electrode tip next to a selected cell. Precise, non-manual positioning of the tip of the microelectrode directly above a single living cell at a distance of a fraction of a micrometre was carried out by taking advantage of topography information available from constant-distance SECM line scans. In the case of catecholamine-releasing chromaffin cells, properly placed SECM tips succeeded to detect amperometrically the release of adrenaline and noradrenaline out of single secretory vesicles upon proper stimulation.
I. Introduction
Microscopic biological cells are the elementary building blocks of living organisms and synchronized activity on a cellular level assures physiological functionality. Even though separated from their native environment, individual enzymatically dissociated or specifically engineered cultured cells largely preserve the ability to transduce and convey a variety of biochemical and biophysical signals, just like those in the body. For that reason, isolated cells became routinely used scaled-down model systems in modern biomedical research and life science for examining, in a controlled and straightforward manner, complex biological processes and functions. However, the proportions of single living cells are small and responses attributable to signal transduction and metabolism are diminutive and often on the sub-second time scale. Viewing and studying these preparations hence requires sophisticated methodologies with adequate sensitivity and high spatio-temporal resolution.In the past few decades remarkable progress has been achieved concerning microscale instrumentation applicable to single cell analysis. Electrochemical detection of transient chemical events at single cells, for example, became accessible through the development of voltammetric microelectrodes with tip diameters in the μm range and millisecond response time.1,2 In particular, constant-potential amperometry and fast-scan cyclic voltammetry at carbon-fibre ultramicroelectrodes has allowed direct detection of neurotransmitters and hormone release out of single secretory vesicles from neuroendocrine cells and neurons.3–5 Scanning electrochemical microscopy (SECM)6,7 was developed by integrating disk-shaped microelectrodes as movable local probes (SECM tips) into 3-D micropositioning devices that were designed for precise perpendicularly moving electrode tips close to a surface of interest (tip approach) and then scanning them vertically while simultaneously recording the tip current as a function of the x–y position (SECM imaging). With the active microelectrode surface located within the electrochemical nearfield, the current response of an amperometrically operated SECM tip may well be influenced e.g. by a substrate-induced generation of a redox active species (substrate generator–tip collector mode), a blockage of diffusion of solution species towards the tip at insulating surface (negative feedback) or a regeneration of tip-consumed dissolved redox species at a conductor (positive feedback). A strong distance dependence of these effects provides the operational principles for electrochemical imaging of the surface topography and conductivity or the interfacial redox activity. Originally applied in surface science for studying processes occurring at solid/liquid interfaces, SECM was rapidly applied also for biological applications, particularly for visualizing chemical gradients in the diffusion layer surrounding cells and tissue.8,9 Representative examples underlining the potential of an SECM-based bioelectroanalysis are imaging the catalytic activity of immobilized enzymes10,11 or antibodies12 and local measurements of the oxygen permeability of cartilage,13 photosynthetic electron transport in guard cells,14 cell respiration,15 the resorptive activity of osteoplasts from the bone matrix in mammals16 or different redox activities of nonmetastatic and metastatic human breast cells.17
In general, SECM measurements are carried out in the constant height mode with probe tips that are scanned in a vertically fixed x–y plane. According to theory, practicable working distances for imaging are just about the diameter of the electroactive disk of the SECM tip since only at this close is the microelectrode tip placed adequately inside the regime of negative/positive feedback or good collection efficiency is guaranteed.18,19 However, the need of a proper working distance is a source of restriction especially when applying the constant height mode for measurement on soft individual living cells. If, for example, a 10 μm diameter microelectrode was brought to working distance near a cell that is adherent to a glass coverslip and about 10 μm high, an enhanced negative feedback will be detected when moving the tip in the direction of the cell since the three-dimensional biological object surely will act as a disturbing diffusional barrier (Fig. 1A). In contrast, feedback will be lost in a lateral scan when tip approach was made exactly on the cell. In either case, SECM images will reflect to some extent the influence of topography and thus variations in the tip response due to changes in (redox) activity of the cell would be suppressed and difficult to identify. Also, the risk of tip crash and cell damage are high with smaller SECM tips essential for improved spatial resolution since they require working distances significantly below the height of cells (Fig. 1B). Forcing a SECM tip in constant-distance mode to follow the surface of the coverslip and contours of cells is the only practical alternative to overcome limitations of scanning in constant height and to facilitate non-destructive tip positioning (Fig. 1C).
 | ||
| Fig. 1 Illustration of constant-height (A, B) and constant distance (C) mode SECM on individual living cells. In the constant-distance mode, the tip-to-sample separation is typically less than about 500 nm. | ||
Constant-distance mode SECM has been established taking advantage of optical and non-optical detection schemes for hydrodynamic shearforces occurring between a liquid/solid interface and SECM tips that vibrate at resonance.20–22 The distance control benefits from shearforce-induced dampening of tip vibration as typically obtained in extreme proximity to the surface. The integrated computer-controlled feedback loop of the device continually compares actual measured oscillation amplitudes with a user-defined set point and responds to deviations due to distance variations by repositioning the tip in such a way that a constant level of damping and non-contact scanning at constant distance of a few hundred nanometres is guaranteed. This opened the opportunity to acquire simultaneously the real sample topography along with the localized electrochemical tip response, which actually promotes interpretation of SECM data and effectively prevents tip crash. Recently, the performance of shearforce-based constant distance SECM was unequivocally demonstrated by successfully operating vibrationable Pt nanoelectrodes for high-resolution SECM imaging.23,24 The system also allowed scanning tips of highly flexible carbon-fibre microelectrodes (∅ 7–8 μm) with a fixed spacing across secretory cells to reveal their topography.25,26 Although the appearance of shearforce interactions is less abrupt over biological cells, there is a clear damping of the vibration of the electrode providing a proper input for the feedback loop of the distance control. Based on the obtained topographical data, line scans allowed the electrode to be placed a submicrometre distance to the membrane of a selected cell before finally detecting single vesicle catecholamine release amperometrically upon appropriate stimulation.
The scope of this paper is two-fold. First, we describe merging a shearforce-based constant-distance mode SECM with an inverted microscope to build an instrument (Bio-SECM) that is most favourable for local electrochemical measurements on single living cells under physiological conditions. The high quality of visual aid with an optical microscope helps to identify healthy cells, supports prepositioning probe tips next to selected cells and hence accelerates experiments. A second part of the work is focused on drastically decreasing the tip diameters of the carbon fibre-based needle-type scanning probes targeting tip dimensions in the 1 μm range. Such miniaturized microelectrodes can elucidate metabolic reactions of cells with higher spatial resolution and are more suitable for the detection on substructures of cells such as dendrites and varicosities.
II. Experimental
Chemicals and solutions
All chemicals were from Sigma-Aldrich (Steinheim, Germany) unless stated otherwise. The external (bath) solution for SECM measurements on chromaffin and fibroblast cells contained (in mM): 100 NaCl, 5 KCl, 5 NaHCO3, 1.2 NaH2PO4, 1.2 MgCl2, 1 CaCl2, 10 glucose, 25 HEPES, pH 7.3. Equimolar NaCl was replaced by 50 mM KCl in the high potassium stimulation solution for amperometric release measurements on chromaffin cells. The electrolyte for cyclic voltammetry (CV) contained 5 mM [Ru(NH3)6]Cl3 in 0.5 M KCl. 0.01 or 0.1 M NaOH solution was the electrolyte for electrochemically etching carbon fibres.Cell preparation
Chromaffin cells were obtained from bovine and mouse adrenal glands and prepared as described elsewhere.27 Freshly isolated cells were plated in a density of about 150–200 cell mm−2 on 10/12 mm glass coverslips, kept in an incubator at 37 °C in 8% CO2 and used on days 1–10 after preparation. Fibroblasts (PCNA) were grown in DMEM-medium supplemented with 10% fetal calf serum and 2 mM glutamine. Routinely, they were trypsinised every 3 days, split one to five, seeded on 10/12 mm glass coverslips in a density of about a 200–300 cells mm−2 and used 24 h later.Vibrationable carbon-fibre microelectrodes (SECM tips)
Disk-shaped carbon microelectrodes were prepared from highly conductive carbon fibres (∅ 7–8 μm, SGL Technik GmbH, Meitingen, Germany) following a previously published procedure.28,29 However, the method was modified in that the carbon-fibre containing glass capillaries (borosilicate glass, od: 1.5 mm, id: 0.75 mm, 100 mm long; Hilgenberg GmbH, Germany) were pulled with a micropipette puller (Narashige Model PP830, Science Products, Hofheim, Germany) into long, needle-type tips required for the shear-force based distance control. To fabricate microelectrodes with a smaller diameter of the electroactive carbon disk, carbon fibres were subjected to an electrochemical etching before insulating their cylindrical face. Electrochemical etching was achieved by inserting pulled carbon-fibre electrodes in the centre of a Ω-shaped Pt foil that served simultaneously as trough for the alkaline solution and anode. A function generator (Model 33120A, Hewlett Packard, Waldbronn, Germany) was employed to expose the carbon fibre to a periodic square wave potential, 3.9 V in amplitude with a frequency of 45 Hz. Etching in 0.01 M NaOH for several minutes led to the known cylindrically etching with the carbon fibre being uniformly decreased in diameter.28 In contrast, applying the procedure in 0.1 M NaOH until the current was observed dropping to zero formed conically shaped tips. Subsequent to etching, the carbon fibres protruding from the tapered glass tips were electrically insulated (“electropainted”) with uniform and thin films of anodic electrophoretic deposition paint (Canguard®, formerly known as Glassophor®, BASF Coatings AG, Münster, Germany). Typically, the paint solution was diluted 1:2 with tri-distilled water. Before experiments, the end of insulated fibres were cut under visual control using a stereomicroscope and a sharp scalpel to expose a fresh disk-shaped carbon surface.SECM instrumentation
All measurements were performed in an electrochemical cell that was home-made simply by gluing a 1.5 mm thick plate of silicon rubber with a rectangular hole (20 mm × 25 mm) to a standard glass slide using a two-component silicon elastomer Sylgard 184 (Dow Corning, Midland, Michigan, USA). The volume of the obtained watertight “thin layer” chamber was about 0.8 ml. For CV and amperometry a low-noise potentiostat (Model VA10, npi electronics GmbH, Tamm/Germany) was used that operated the carbon-fibre microelectrode (SECM tip) as working and a Ag/AgCl pellet as pseudo reference electrode. For SECM measurements, the electrochemical cell was mounted on a x,y,z-micropositioning stage that was driven by computer-controlled stepper motors (SPI Robot Systems Oppenheim, Germany) having a nominal resolution of 10 nm per microstep. For gentle tip approach (z-movement) and fast readjustments of the z-height during scanning, an extra piezo-driven table (Owis, Staufen, Germany) was used. Like all other components of the constant-distance SECM, the micropositioning device was fixed to the base plate of the inverted microscope (Axiovert 25C, Carl Zeiss Jena, Germany). Through a front port, the microscope was further equipped with a video camera (IDS Imaging Development System GmbH, Obersulm, Germany). The components to complete a functioning optical shearforce distance control are (i) a piezoelectric tube for tip agitation (PST500/5/15, Piezomechanik Pickelmann, München, Germany), (ii) a split photodiode (Spot 4D, LASER 2000, Weßling, Germany), (iii) a low-power LASER (CDM 14S/S70/1, Atos, Pfungstadt, Germany) and (iv) a lock-in amplifier (Model 5210, Signal Recovery, Bad Wildbad, Germany). The LASER and photodiode were fixed to micrometer-screw driven coarse positioning tables (Owis, Staufen, Germany) to allow easy optical alignment. The whole set-up was placed on a vibration-damping table and located in a Faraday cage for noise reduction. A PC in combination with a Windows software programmed in Microsoft Visual Basic 3.0 (Microsoft, Unterschleißheim, Germany) was used for the control of all system parameters and for data acquisition.III. Results
The design of the Bio-SECM not only had to be rigorously tailored to the limited space available on an inverted microscope but also had to meet the needs of the constant distance feedback loop with an integrated optical detection of shearforces. With this system, a LASER beam has to be focused onto the vibrating SECM tip and the resulting Fresnel diffraction pattern projected on a split photodiode, which transmitted current values of the vibration amplitude to the feedback loop by means of lock-in technique. Accordingly, it is essential to keep the SECM tip, the LASER beam and the split photodiode permanently in perfect alignment while operating the constant distance mode, a prerequisite that only can be achieved by laterally scanning the sample instead of the tip. Fig. 2 is a scheme that shows the arrangement of the individual components of the constant distance mode SECM, as they are placed on the microscopes base-plate. The holder for the electrochemical chamber containing cells on glass coverslips is connected to a high precision x, y, z micropositioning device that allows movements in all directions with nanometer resolution and is firmly attached to the right of the base plate. At the opposite side, a coarse manipulator is used for carrying a special holder for the SECM tip and the piezoelectric tube for tip agitation. The manipulator is practical for centring the microelectrode above the lens of the objective and lowering it into electrolyte to about 0.5 mm distance to surface. After such a prepositioning, the tip is brought to proper alignment with the beam source and photodiode, which are individually fixed to coarse manipulators on the front (LASER) and back (photodiode) of the base plate.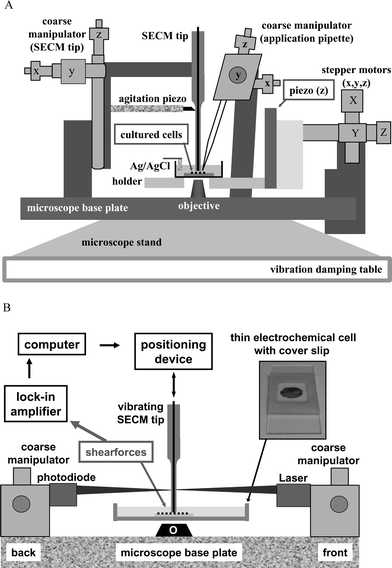 | ||
| Fig. 2 A. Schematic representation of a biological scanning electrochemical microscope (Bio-SECM) built around an inverted optical microscope. B. Block diagram of the shearforce based distance control with an optical detection of the vibration amplitude of an oscillating SECM tip. | ||
The difference signal of the photodiode is the optical measure of changes in tip vibration and provides the distance-dependent input for the feedback loop that maintains constant tip-to-sample separation. Therefore, its intensity and integrity is of critical importance for the quality of an established constant distance mode of scanning. Guiding the LASER beam and diffracted light as previously described through transparent electrochemical cells with parallel (glass) windows and the electrolyte could cause disturbing deterioration of the optical signal. Therefore, a “thin-layer” electrochemical cell was used instead and offered signal enhancement simply by operating the LASER above the electrolyte solution in air only.
The key element for high-performance constant distance mode SECM is the highly flexible needle-type tip electrode that can be employed as vibrationable scanning probe. For combined topographic and electrochemical measurements on living cells, the stiffness of, at least, the very end of the microelectrode body should not be excessively high as otherwise the force of interaction between the tip and sample will deform and, in the worst case, even destroy the soft object. Until now, only disk-shaped carbon-fibre microelectrodes with polymer-insulated carbon fibres protruding from the tapered tips of pulled glass pipettes have been described as suitable for successfully operating a distance control with optical detection of shearforces on cells without damaging them.25,26 Typically, the LASER spot was focussed on the end of such an SECM tip, directly on the electropainted carbon fibre. However, their structural dimensions are very small (<10 μm) and the resulting diffraction patterns for that reason were extremely weak. Although used with success, this limitation made a proper adjustment of the feedback loop difficult to achieve and brought the system for the optical detection of shearforces close to the limits. Hence, it was found more suitable to focus the LASER not on the thin carbon fibre but alternatively above the fibre/glass junction on the tapered glass. This results in a stronger diffraction pattern, which significantly increased the difference current from the split photodiode and thus the stability of the optically operated feedback mechanisms controlling the tip-to-sample distance. However, with the LASER spot located on the glass structure far away from the site of interaction, the flexibility of the tapered glass tips had to be optimised by varying its length and thickness and the carbon fibre had to be kept short to attain appropriate vibration characteristics for detecting shearforces on soft cells.
Fig. 3 displays a series of scanning electron microscope (SEM) images of the shapes of highly flexible disk-shaped carbon-fibre microelectrodes as used in Bio-SECM as vibrationable SECM tips for measurements on individual fibroblast and chromaffin cells. Typically, the length of electropainted carbon fibre protruding from the glass tip was kept between 200 to 500 μm. The diameter of the tapered glass tip near the glass/fibre junction was adjusted to be about 50 μm by choosing proper parameters for the pulling procedure. Due to the very small opening angle of the tapered glass tips formed, the diameter of the glass structure remained in the order of 50–100 μm, even at several millimetres above the junction (not shown). This feature was considered important since for establishing a properly working shearforce-based distance control the LASER beam had to be located about 5 mm above the tip of the electrode to ensure that the light path can be guided above the “thin layer” electrochemical cell through air.
 | ||
| Fig. 3 A–C. SEM images at different magnifications of a representative conically etched and electropainted carbon-fibre microdisk electrode as used in the Bio-SECM with an optical detection of shearforces. D. The tip of a conventionally-sized (unetched) carbon-fibre microdisk electrode. Of note, scale bars in C and D are drawn equal in size to allow an easy direct comparison of the tip size of etched and unetched microelectrodes. | ||
As can be seen from Fig. 3C and D, conical electrochemical etching allowed a decrease of the diameter of the electroactive carbon tip 4 fold (from about 8 μm of the original fibre to about 2 μm for an etched fibre). The cylindrical electrochemical etching procedure that was proposed earlier28 can also be used for the production of miniaturised microelectrodes. However, microelectrodes produced through this route do not have the mechanical stability for the transmission of shearforces from their tip to the glass.
Cyclic voltammetry of 5 mM [Ru(NH3)6]Cl3 in 0.5 M KCl was used to evaluate the electrochemical properties of conically etched carbon fibre microelectrodes that were meant to be used for applications as flexible SECM tips. Usually, the obtained cyclic voltammograms displayed reproducibly the characteristic sigmoidal response expected for disk-shape microelectrodes, with no significant hysteresis (Fig. 4). As described earlier28 and based on a study by Zhao et al.,30 the size of thinly-insulated, electropainted carbon-fibre microelectrodes can be calculated from the steady-state diffusion-limited currents using the equation ilim = 4.83nFDCr with r the radius of the electrode and all other terms having their usual electrochemical meaning. In good agreement with the SEM observations, the diameter of the electroactive carbon disk for unetched electrodes was calculated to be about 8 μm while the smaller, conically etched carbon-fibre microelectrode exposed radii of about 1.6–2 μm.
![Cyclic voltammograms obtained using a conically etched and unetched carbon-fibre microelectrode. (electrolyte: 5 mM [Ru(NH3)6]Cl3 in 0.5 M KCl; scan rate: 100 mV s−1).](/image/article/2004/CP/b405233a/b405233a-f4.gif) | ||
| Fig. 4 Cyclic voltammograms obtained using a conically etched and unetched carbon-fibre microelectrode. (electrolyte: 5 mM [Ru(NH3)6]Cl3 in 0.5 M KCl; scan rate: 100 mV s−1). | ||
The completion of the Bio-SECM set-up and access to flexible, carbon fibre-based SECM tips paved the way to apply the instrument and the novel probes for constant-distance SECM measurements on individual living cells in order to test their suitability for biological studies. To begin with, SECM imaging was performed in the constant distance mode of operation on fibroblast cells. The inverted microscope was employed for identification of cells in good physical shape and positioning the SECM tip close to a cell of choice before tip approach.
A typical example of a microscopic image used to recognize healthy cells in a larger population is shown in Fig. 5. The tip of the microelectrode was also visible and thus could be easily positioned right next to a well-adherent cell.
 | ||
| Fig. 5 Video camera image of a population of fibroblast cells and the tip of a 8 μm diameter carbon-fibre microelectrode obtained with the inverted microscope of the Bio-SECM. | ||
Knowing the exact position of the SECM tip relative to cells of interest, sets of shearforce-based approach curves (plots of the difference signal of the photodiode as measured with the lock-in amplifier vs. tip z-height, not shown) was recorded with the tip vibrating at resonance frequency and either moving slowly towards the glass coverslip or the deformable membrane of a soft cell. A sudden drop in the response of the photodiode was always observed when the tip of an oscillating carbon-fibre microelectrode came into close proximity to the solid glass surface. This was expected given the fact that shear-force induced dampening of the tip vibration only takes place at distances of much less than one micrometer. On the contrary, a sharp decay did not occur while approaching soft fibroblast cells. Instead, the optical signal decreased gradually with the tip-to-sample distance. It seems that the elasticity of the cell membrane made the cell buckle and deform under the influence of shearforce interaction. Apparently, a cell deformation decreased the level of vibration dampening, as compared with the shearforce contact with a hard (glass) surface.
The Bio-SECM was then further used for topography measurements on adherently growing fibroblast cells. Before imaging, approach curves were recorded in the vicinity of a selected cell and used to adjust the parameters of the shearforce based feedback loop constantly regulating the distance of the SECM tip to the sample surface. While travelling across the cell from the left to the right or vice versa, changes were recorded in the voltage applied to the piezoelectric z-positioning element that are due to shearforce-induced tip-displacements, converted into distances (z-height) and plotted as a function of tip position in x- and y-direction. Fig. 6 represents a line scan across a selected cell reflecting its topography. Obviously, the shearforce interaction between the microelectrode tip and the cell was not destructive as the cell remained intact and in place after the scan. This is an indication that the flexibility of the carbon-fibre microelectrodes and the sensitivity of the optically controlled feedback distance control are adequate for visualizing soft biological samples.
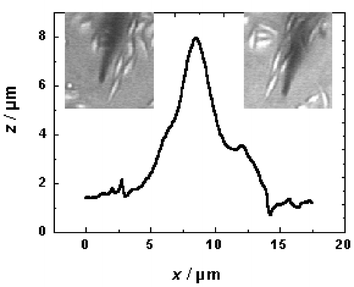 | ||
| Fig. 6 Topographical imaging of a soft biological sample with the Bio-SECM. The line scan across a fibroblast cell was obtained in the constant-distance mode of operation with an optical detection of shearforces and a 2 μm diameter carbon fibre microdisk electrode acting as the scanning probe. The insets show the relative position of the SECM tip before (left) and after (right) completion of a full scan. | ||
The optically controlled shearforce-based distance control of the Bio-SECM was finally employed to position the tip of carbon-fibre microelectrodes precisely above the outer membrane of individual adrenal chromaffin cells that are known to secrete electrochemically detectable epinephrine and norepinephrine via single-vesicle exocytosis. A line scan crossways the cell was performed and the scan was stopped in the middle of the cell diameter, thus positioning the microelectrode tip at about a few hundred nanometres above the centre of a single cell. Subsequent to tip positioning, an amperometric detection of stimulated catecholamine release was induced by application of high K+.
Fig. 7 is a comparison of two amperometric recordings of depolarisation-evoked catecholamine secretion from chromaffin cells that were acquired with a conventionally-sized, unetched (A) and a miniaturised conically etched (B) carbon-fibre microelectrode, respectively. In good agreement with the literature, the release of epinephrine and norepinephrine out of the intracellular storage vesicles has been detected during exocytosis as spike-like current transients in either case.
 | ||
| Fig. 7 Representative examples of amperometric recordings obtained at individual chromaffin cells after application of high potassium stimulation solution. Tip positioning above cells was performed taking advantage of the shearforce-based constant distance mode of the Bio-SECM. For measurements, the carbon fibre microelectrodes (A: unetched, 8 μm diameter; B: conically etched, 2 μm diameter) were kept at a constant voltage of 700 mV vs. Ag/AgCl with 1000 Hz as data acquisition rate. | ||
The visibly lower number of amperometric spikes that were typically observed at etched carbon-fibre microelectrodes can be explained by the reduced size of their electroactive carbon disk leading to a lower collector efficiency at concomitantly increased spatial resolution. Actually, the surface area of an etched 2 μm diameter carbon fibre microelectrode is about 16 times smaller than that of an 8 μm diameter unetched microelectrode. Thus, the number of vesicles accessible by the etched electrode for the electrochemical detection of exocytosis is considerably smaller leading to the smaller number of spikes observed. However, the smaller size of the etched microelectrode, in principle, allows local measurements at different points on the membrane of one and the same secretory cell which could provide improved spatial details about secretory activity.
Conclusion
An inverted optical microscope was modified to accommodate all the components needed for operating a constant-distance mode SECM with a shearforce-based optical distance control. The resulting Bio-SECM was used in combination with novel, highly flexible, conically etched carbon-fibre microelectrodes for topographical and electrochemical measurements on individual living cells. SECM line scan measurements allowed a precise, non-manual placement of the tip of the microelectrode right above the soft biological samples at distances of a fraction of a micrometre. In the case of secretory chromaffin cells, the properly positioned SECM tip succeeded to detect, amperometrically, the release of catecholamines out of single exocytotic vesicles.Acknowledgements
This work was in part financially supported by the Deutsche Forschungsgemeinschaft (DFG) (Schu 929/5–1) and the Ministry of Education and Research, Germany (BMBF) in the framework of its program “Nanobiotechnologie” (AZ NBT066). The authors are grateful to Prof. Erwin Neher and Dr Jakob Sørensen, Membranbiophysik, Max-Planck-Institut für Biophysikalische Chemie, Göttingen, Germany for providing adrenal chromaffin cells and to Dr Andrea Blöchl, Molekulare Neurobiochemie, Ruhr-Universität Bochum, Germany for the preparation of fibroblast cultures. Furthermore, we would like to thank Dr Thomas Erichsen (Sensolytics GmbH, Bochum) for help in adapting the SECM software and the workshop staff at the Faculty of Chemistry at the Ruhr-Universität Bochum, especially Armin Lindner, for their helpful input in building up the described SECM instrument.References
- E. R. Travis and R. M. Wightman, Ann. Rev. Biophys. Biomol. Struct., 1998, 27, 77 CrossRef CAS.
- G. Y. Chen and A. G. Ewing, Crit. Rev. Neurobiol., 1997, 11, 59 Search PubMed.
- R. H. Chow, L. von Rüden and E. Neher, Nature, 1992, 356, 60 CrossRef CAS.
- R. M. Wightman, J. A. Jankowski, R. T. Kennedy, K. T. Kawagoe, T. J. Schroeder, D. J. Leszczyszyn, J. A. Near, E. J. Diliberto and O. H. Viveros, Proc. Natl. Acad. Sci. USA, 1991, 88, 10754 CAS.
- D. Bruns and R. Jahn, Nature, 1995, 377, 62 CAS.
- R. C. Engstrom, M. Weber, D. J. Wunder, R. Burgess and S. Winquist, Anal. Chem., 1986, 58, 844 CrossRef CAS.
- A. J. Bard, F.-R. F. Fan, J. Kwak and O. Lev, Anal. Chem., 1989, 61, 132 CrossRef CAS.
- B. R. Horrocks and G. Wittstock, in Scanning Electrochemical Microscopy, ed. A. J. Bard, M. V. Mirkin, Marcel Dekker, New York, 2001, p. 445 Search PubMed.
- A. L. Barker, M. Gonsalves, J. V. MacPherson, C. J. Slevin and P. R. Unwin, Anal. Chim. Acta, 1999, 385, 223 CrossRef CAS.
- G. Wittstock and W. Schuhmann, Anal. Chem., 1997, 69, 5059 CrossRef CAS.
- S. Gàspàr, M. Mosbach, L. Wallmann, T. Laurell, E. Csöregi and W. Schuhmann, Anal. Chem., 2001, 73, 4254 CrossRef CAS.
- G. Wittstock, K. J. Yu, H. B. Halsall, T. H. Ridgway and W. R. Heinemann, Anal. Chem., 1995, 67, 3578 CrossRef CAS.
- M. Gonsalves, A. L. Barker, J. V. MacPherson, P. R. Unwin, D. O'Hare and C. P. Winlove CP, Biophys. J., 2000, 78, 1578 CrossRef CAS.
- M. Tsionsky, Z. G. Cardon, A. J. Bard and R. B. Jackson, Plant Physiol., 1997, 113, 895 CAS.
- T. Yasukawa, T. Kaya and T. Matsue, Chem. Lett., 1999, 9, 975 CrossRef.
- C. E. M. Berger, H. Rathod, J. I. Gillespie, B. R. Horrocks and H. K. Datta, J. Bone Miner. Res., 2001, 16, 2092 CAS.
- W. J. Feng, S. A. Rotenberg and M. V. Mirkin, Anal. Chem., 2003, 75, 4148 CrossRef CAS.
- J. Kwak and A. J. Bard, Anal. Chem., 1989, 61, 1221 CrossRef CAS.
- A. J. Bard, F.-R. F. Fan and M. V. Mirkin, in Electroanalytical Chemistry, ed. A. J. Bard, Marcel Dekker, New York, 1994, p. 243 Search PubMed.
- M. Ludwig, C. Kranz, W. Schuhmann and H. E. Gaub, Rev. Sci. Instrum., 1995, 66, 2857 CrossRef CAS.
- A. Hengstenberg, C. Kranz and W. Schuhmann, Chem. Eur. J., 2000, 6, 1547 CrossRef CAS.
- B. Ballesteros Katemann, A. Schulte and W. Schuhmann, Chem. Eur. J., 2003, 9, 2025 CrossRef.
- B. Ballesteros Katemann, A. Schulte and W. Schuhmann, Electroanalysis, 2004, 16, 60 CrossRef CAS.
- M. Etienne, A. Schulte and W. Schuhmann, Electrochem. Commun., 2004, 6, 288 CrossRef.
- A. Hengstenberg, A. Blöchl, I. D. Dietzel and W. Schuhmann, Angew. Chem. Int. Ed., 2001, 40, 905 CrossRef CAS.
- A. Hengstenberg, I. D. Dietzel, A. Blöchl and W. Schuhmann, BIOForum Forsch. Entwicklung, 1999, 22, 595 Search PubMed.
- G. Nagy, U. Matti, R. B. Nehring, T. Binz, J. Rettig, E. Neher and J. B. Sørensen, J. Neurosci., 2002, 22, 9278 CAS.
- A. Schulte and R. H. Chow, Anal. Chem., 1996, 68, 3054 CrossRef CAS.
- A. Schulte and R. H. Chow, Anal. Chem., 1998, 70, 985 CrossRef CAS.
- G. Zhao, D. M. Giolando and J. R. Kirchhoff, Anal. Chem., 1995, 67, 2592 CrossRef CAS.
| This journal is © the Owner Societies 2004 |
