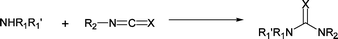Self-indicating amine scavenger resins
Jin Ku
Cho
a,
Peter D.
White
b,
Wolfgang
Klute
c,
Tony W.
Dean
d and
Mark
Bradley
*a
aCombinatorial Centre of Excellence, School of Chemistry, University of Southampton, Highfield, Southampton, UK SO17 1BJ. E-mail: mb14@soton.ac.uk; Fax: +44 (0)23 8059 6766; Tel: +44 (0)23 8059 3598
bMerck Biosciences Inc., Boulevard Industrial Park, Padge Road, Nottingham, UK NG9 2JR
cPfizer Global Research & Development, Ramsgate Road, Sandwich, UK CT13 9NJ
dGlaxoSmithKline, Discovery Research, Gunnels Wood Road, Stevenage, UK SG1 2NY
First published on 27th January 2004
Abstract
Self-indicating methylisocyanate resin, which functions as both a scavenger and an indicator for amines, was used for in-situ reaction monitoring and purification of a urea based library.
One the major advantages of solid-phase synthesis is that it avoids much of the effort necessary for purification that follows conventional solution phase synthesis. However, a great many more reactions have been optimized and documented for solution-phase synthesis, while solution-phase chemistry avoids resin attachment and cleavage steps (although these can be simply considered as purely protection and deprotection steps of an insoluble protecting group!). In addition, reaction progress as well as the identity and purity of products may be analyzed and established by standard chromatographic and spectroscopic means. Parallel combinatorial methods have therefore been widely applied in the solution-phase synthesis of compound libraries.1 This includes solution-phase libraries prepared using a variety of resin-based systems such as polymer-immobilized reagents, catalysts or scavengers.2 Many scavenger resins have been employed to remove excess reagents and by-products from crude reaction products,3 and one of the most popular is methylisocyanate resin which is commonly utilized for the sequestration of primary and secondary amines. This amine scavenger resin has for example been applied for the purification of urea-based libraries.4 In general, a 1.5–3.0 fold excess of the resin is used for 1–18 h to remove excess amines. In order to efficiently use the expensive resin and conveniently determine the completion of each different reaction in a solution-phase parallel combinatorial library, simple and reliable monitoring methods are required as conventional chromatographic analyses are simply too laborious to be applied to large libraries.
Here, we wish to report on a novel scavenger resin, which not only scavenges the amine from solution but also indicates its presence. The dye used was bromophenol blue, which is blue in the presence of amines, and resin-bound bromophenol blue has been successfully employed in monitoring amines in solution.5 This dye was derivatized via a Suzuki reaction between bromophenol blue and 4-carboxyphenylboronic acid† and then coupled with mono tert-Boc protected diaminopentane with 1 using TFFH (tetramethylfluoroformamidinium hexafluorophosphate) to give 2 in 85% yield. After deprotection of the tert-Boc group with 50% TFA in DCM,‡ the compound was attached to methylisocyanate polystyrene resin with ∼5% of total sites loaded (Scheme 1).§,¶ When self-indicating scavenger resin (0.20 mmol of resin-bound isocyanate) was added to remove amine after urea formation with n-butylamine (0.25 mmol) and phenylisocyanate (0.20 mmol), the resin immediately turned blue due to excess amine (Fig. 1a). As the amine in solution was removed (quenched) by the resin-bound isocyanate, the resin's color gradually changed to yellow (Fig. 1b–1d). After filtration and evaporation of solvent, the crude product was obtained in 98% yield and 99% purity.
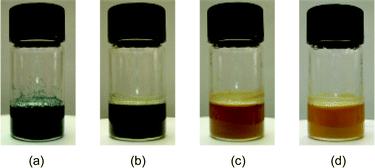 | ||
| Fig. 1 Color change of self-indicating methylisocyanate polystyrene resin used during the purification of urea: (a) t = 0 h, (b) t = 1 h, (c) t = 2 h, (d) t = 3 h. | ||
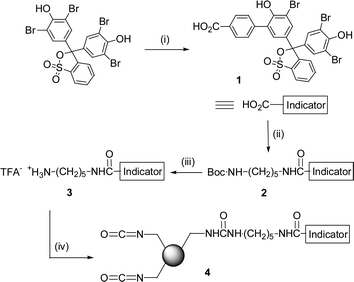 | ||
| Scheme 1 Preparation of self-indicating methylisocyanate resin. Reagents and conditions: (i) 4-carboxyphenylboronic acid, Pd(OAc)2, K3PO4, (n-Bu)4NBr, DMF, 100 °C, 33%; (ii) tBoc-NH-(CH2)5-NH2, TFFH, TEA, DMF, 85%; (iii) TFA, DCM; (iv) methylisocyanate polystyrene resin, TEA, DMF. | ||
A small solution-phase library was prepared using a 24 well microplate. Six amines including one secondary amine and three isocyanates and one isothiocyanate were used as building blocks. In each column of the plate was placed the same amine (0.25 mmol) and in each row was added isocyanate (0.20 mmol). After shaking for 2 h, the self-indicating scavenger resin (0.10 mmol each) was added to each well. The resins immediately became blue due to excess amine but gradually turned yellow as the amines were scavenged by isocyanate resin. After 1 h, about two thirds of the 24 reaction wells had turned a yellowish color (Fig. 2b). Although the blue color of most reaction wells disappeared after about 3 h, the colors of three wells (entries 2c, 3b, 3d) still showed a reddish blue (Fig. 2c). To these three wells were added additional self-indicating scavenger resin (0.10 mmol) and they were shaken for 2 h to completely eliminate the blue color (Fig. 2d). After filtration and evaporation, the yields and purities of the 24 ureas were determined. The results are summarized in Table 1. All the reactions gave high purities (91–99%) even in cases where yields were not so high. In this experiment, the character of the amine had little influence upon purification/scavenging kinetics but the three reactions that required more scavenger resin showed lowest yields.
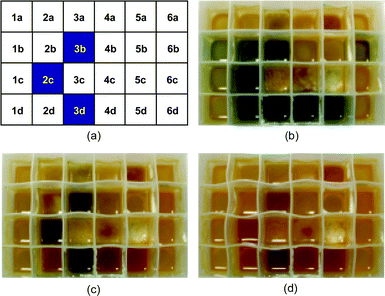 | ||
| Fig. 2 Color change of library according to time: (a) position of each compound in 24 well microplate, (b) t = 1 h, (c) t = 3 h, (d) more scavenger added. | ||
| Entry | R1, R1′ | R2 | X | Yield (%) | Puritya (%) |
|---|---|---|---|---|---|
| a Purities were determined by integration (λ = 250 nm) of the crude products by HPLC and confirmed by LC/MS. | |||||
| 1a | Isopentyl, H | Bn | O | 99 | 99 |
| 2a | Bn, H | Bn | O | 97 | 95 |
| 3a | 4-MeOBn, H | Bn | O | 99 | 96 |
| 4a | 4-CF3Bn, H | Bn | O | 98 | 94 |
| 5a | Ph2CH, H | Bn | O | 98 | 95 |
| 6a | CH2(CH2)3CH2 | Bn | O | 97 | 99 |
| 1b | Isopentyl, H | 4-MeOBn | O | 95 | 99 |
| 2b | Bn, H | 4-MeOBn | O | 87 | 96 |
| 3b | 4-MeOBn, H | 4-MeOBn | O | 48 | 91 |
| 4b | 4-CF3Bn, H | 4-MeOBn | O | 90 | 95 |
| 5b | Ph2CH, H | 4-MeOBn | O | 99 | 95 |
| 6b | CH2(CH2)3CH2 | 4-MeOBn | O | 87 | 98 |
| 1c | Isopentyl, H | 2-ClBn | O | 100 | 99 |
| 2c | Bn, H | 2-ClBn | O | 66 | 93 |
| 3c | 4-MeOBn, H | 2-ClBn | O | 99 | 96 |
| 4c | 4-CF3Bn, H | 2-ClBn | O | 100 | 96 |
| 5c | Ph2CH, H | 2-ClBn | O | 99 | 94 |
| 6c | CH2(CH2)3CH2 | 2-ClBn | O | 99 | 99 |
| 1d | Isopentyl, H | Bn | S | 99 | 99 |
| 2d | Bn, H | Bn | S | 83 | 96 |
| 3d | 4-MeOBn, H | Bn | S | 62 | 94 |
| 4d | 4-CF3Bn, H | Bn | S | 75 | 98 |
| 5d | Ph2CH, H | Bn | S | 70 | 95 |
| 6d | CH2(CH2)3CH2 | Bn | S | 87 | 99 |
In summary, self-indicating scavenger resins were successfully used for in-situ reaction monitoring and purification during the synthesis of a library of ureas and provide an ideal way of visually following arrays of reactions.
We thank the Combinatorial Centre of Excellence partners: GSK, AstraZeneca, Pfizer, Roche, Eli-Lilly, Organon, NovaBiochem/Merck, Amersham/Nycomed Amersham and Evotec OAI and EPSRC (JIF initiative).
Notes and references
- (a) T. Carrell, E. A. Wintner, A. Bashir-Hashemi and J. Rebek Jr., Angew. Chem., Int. Ed., 1994, 33, 2059 CrossRef; (b) D. L. Boger, D. M. Tarby, P. L. Myers and L. H. Caporale, J. Am. Chem. Soc., 1996, 118, 2109 CrossRef CAS; (c) D. P. Curran and N. Hoshino, J. Org. Chem., 1996, 61, 6480 CrossRef CAS; (d) T. A. Keating and R. W. Armstrong, J. Am. Chem. Soc., 1996, 118, 2574 CrossRef CAS.
- (a) R. J. Booth and J. C. Hodges, Acc. Chem. Res., 1999, 32, 18 CrossRef CAS; (b) J. J. Parlow, R. V. Devraj and M. S. South, Curr. Opin. Chem. Biol., 1999, 3, 320 CrossRef CAS; (c) J. Eames and M. Watkinson, Eur. J. Org. Chem., 2001, 1213 CrossRef CAS.
- (a) S. W. Kaldor, M. G. Siegel, J. E. Fritz, B. A. Dressman and P. J. Hahn, Tetrahedron Lett., 1996, 37, 7193 CrossRef CAS; (b) S. W. Kaldor, J. E. Fritz, J. Tang and E. R. McKinney, Bioorg. Med. Chem. Lett., 1996, 6, 3041 CrossRef CAS; (c) R. J. Booth and J. C. Hodges, J. Am. Chem. Soc., 1997, 119, 4882 CrossRef CAS; (d) M. W. Creswell, G. L. Bolton, J. C. Hodges and M. Meppen, Tetrahedron, 1998, 54, 3983 CrossRef CAS; (e) C. Hulme, L. Ma, J. Romano and M. Morrissette, Tetrahedron Lett., 1999, 40, 7925 CrossRef CAS; (f) I. D. Stevenson, A. L. Smith, S. Lewis, S. G. Michie, J. G. Neduvelil, S. Patel, R. Marwood, S. Patel and J. L. Castro, Bioorg, Med. Chem. Lett., 2000, 10, 2697 CrossRef.
- B. A. Dressman, U. Singh and S. W. Kaldor, Tetrahedron Lett., 1998, 39, 3631 CrossRef CAS.
- J. K. Cho, P. D. White, W. Klute, T. W. Dean and M. Bradley, J. Comb. Chem., 2003, 5, 632 CrossRef CAS.
Footnotes |
| † Synthesis of1: Bromophenol blue (2.0 g, 3.0 mmol), 4-carboxyphenylboronic acid (3.6 mmol, 1.2 equiv.), potassium phosphate (9.0 mmol, 3.0 equiv.), tetrabutylammonium bromide (3.0 mmol, 1.0 equiv.), and palladium(II) acetate (2.0 mol%) were placed in a reactor capped with a septum and gently sparged with argon for 10 min. DMF (10 mL) was added and the reaction mixture was stirred under argon at 110 °C for 18 h. The resulting mixture was treated with saturated KHSO4, extracted with EtOAc, dried over Na2SO4, and evaporated under reduced pressure to afford a reddish oil. The product was isolated by column chromatography on silica gel (chloroform : methanol : acetic acid = 5 : 1 : 0.06) to give 1 in 33% yield: 1H NMR (400 MHz, 5% CF3CO2D in CD3CN): δ = 8.03 (d, J = 8.4 Hz, 2H), 7.95 (d, J = 6.8 Hz, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.74 (t, J = 7.8 Hz, 1H), 7.58 (d, J = 7.6 Hz, 1H), 7.54 (d, J = 8.4 Hz, 2H), 7.47 (d, J = 2.4 Hz, 1H), 7.45 (s, 2H), 7.17 (d, J = 2.0 Hz, 1H); calculated mass for C26H15Br3O7S: 711.2, found: ESI-MS, m/z, negative [M − H]−: 708.4, 710.6. |
| ‡ Synthesis of3:1 (360 mg, 0.5 mmol) was dissolved in DMF (5 mL). Triethylamine (5 mmol, 10 equiv.), mono tert-Boc protected diaminopentane (0.75 mmol, 1.5 equiv.), and TFFH (1.0 mmol, 2.0 equiv.) were added and the reaction mixture was stirred for 18 h. The resulting mixture was treated with saturated KHSO4, extracted with EtOAc, dried over Na2SO4, and evaporated under reduced pressure to afford a reddish oil. The product was isolated by column chromatography on silica gel (chloroform : methanol = 3 : 1) to give 2 in 85% yield: calculated mass for C36H35Br3N2O8S: 895.5, found: ESI-MS, m/z, negative [M − H]−: 893.5, 895.0. To deprotect tert-Boc group, 2 was treated with 50% TFA in DCM for 4 h. TFA and DCM were removed by nitrogen bubbling and the resulting residue was precipitated with cold diethyl ether and filtered to give 3 (orange solid): 1H NMR (400 MHz, 5% CF3CO2D in CD3CN): δ = 7.95 (d, J = 7.2 Hz, 1H), 7.84–7.80 (m, 3H), 7.75 (t, J = 7.6 Hz, 1H), 7.59–7.54 (m, 3H), 7.47–7.45 (m, 3H), 7.18 (d, J = 2.0 Hz, 1H), 3.42 (t, J = 6.0 Hz, 2H), 2.94 (t, J = 6.0 Hz, 2H), 1.71–1.60 (m, 4H), 1.41 (p, J = 8.0 Hz, 2H); calculated mass of C31H27Br3N2O6S: 795.4, found: ESI-MS, m/z, positive [M + H]+: 795.0, 797.4, negative [M − H]−: 792.6, 794.4. |
| § Preparation of4: Methylisocyanate polystyrene resin (3.0 g, 4.5 mmol, 0.15 mmol of isocyanate/g resin) was added to 3 (0.23 mmol, 5% of total loading capacity of methylisocyanate resin), triethylamine (0.69 mmol, 3.0 equiv.) in DMF (20 mL) and the suspension was shaken for 1 h. The resin was filtered and washed with 5% acetic acid in DCM, 5% triethylamine in DCM, and DMF by turns until no blue color was present in the washing. |
| ¶ IR absorption at 2260 cm−1 designates the CO stretching peak of resin-bound isocyanate. No change was found between methylisocyanate resin and indicator–methylisocyanate resin. |
| This journal is © The Royal Society of Chemistry 2004 |