Synthesis of poly(para-phenylenevinylene) rotaxanes by aqueous Suzuki coupling†
Jun
Terao
,
Andrew
Tang
,
Jasper J.
Michels
,
Alexander
Krivokapic
and
Harry L.
Anderson
*
Department of Chemistry, University of Oxford, Dyson Perrins Laboratory, South Parks Road, Oxford, UK OX1 3QY. E-mail: harry.anderson@chem.ox.ac.uk; Fax: 44 1865 275674; Tel: 44 1865 275704
First published on 27th November 2003
Abstract
PPV-based polyrotaxanes have been prepared by coupling vinyl boronic acids to aryl iodides in the presence of cyclodextrins, and the crystal structure of a [2]rotaxane of this type has been determined.
Poly(para-phenylenevinylene)s, PPVs, and oligo(para-phenylenevinylene)s, OPVs, are among the most extensively studied organic semiconductors because their efficient luminescence and charge-transport properties1 lead to applications in light emitting diodes,2 sensors3 and lasers.4 Recently we have shown that the fluorescence quantum yields, electroluminescence efficiencies and chemical stabilities of conjugated π-systems can be enhanced by molecular-scale encapsulation to form “insulated molecular wires”.5–9 Our encapsulation-strategy is to thread the π-system through a series of cyclodextrins using hydrophobic binding, then to attach bulky end-groups to form a polyrotaxane. Biphenyl-linked conjugated polyrotaxanes are conveniently prepared by the aqueous Suzuki coupling of aryl iodides and aryl boronic acids.5–8 Here we demonstrate that this approach can be extended to vinyl boronic acids, providing a route to PPV-based insulated molecular wires. We also report the crystal structure of an OPV-based [2]rotaxane which sheds light on the mechanism of inter-strand charge-transport in these materials.
The Suzuki coupling of vinyl boronic acids is rarely conducted in water,10 so our first task was to test conditions for this reaction. When a solution of diboronic acid 1 (0.07 M) in D2O containing NaOH (0.15 M) and Na2CO3 (0.15 M) was treated with two equivalents of sodium iodoisophthalate 2, in the presence of palladium(II) acetate (1 mol%) under nitrogen at 20 °C, a rapid reaction was observed by 1H NMR giving 3 as the only detectable product (>90% yield within 2 h). When this reaction was repeated in the presence of α-cyclodextrin, we observed formation of the [2]rotaxane 3⊂α-CD (Scheme 1). The yield of rotaxane was improved by increasing the temperature to 50 °C. Under these conditions a five-fold excess of α-cyclodextrin gave a crude reaction mixture with a 3 : 1 ratio of 3⊂α-CD : 3, from which pure 3⊂α-CD was isolated in 46% yield.† The structure of this rotaxane was confirmed by 2D NMR, ESI MS and X-ray crystallography.†‡ Hydrated crystals of 3⊂α-CD were grown from water, by cooling a hot saturated solution. The asymmetric unit contains two molecules of 3⊂α-CD, as shown in Fig. 1, and about 24 water molecules. In both rotaxane molecules, the α-cyclodextrin is displaced from the centre of the distyrylbenzene core, with its wider 2,3-rim cradling the central phenylene unit. The distyrylbenzene π-systems are essentially planar, and the phenylene–vinylene torsion angles are unaffected by the proximity of the threaded cyclodextrin (8–14° inside cavity; 3–14° outside cavity). The data are not good enough to reliably locate the hydrogen atoms, but the abundance of short O⋯O distances reveals a dense mesh of H-bonds. The main rotaxane–rotaxane intermolecular contacts are π–π stacking of the isophthalic acid end groups, CH⋯π interactions between isophthalic acid units and external cyclodextrin CH protons (H1, H2 and H4), H-bonding between cyclodextrin OH groups (O3–O3, O3–O2, O6–O2 and O6–O6) and H-bonding of CO2H to cyclodextrin OH groups at O3.§ Every isophthalic acid end-group is π-stacked with another isophthalic acid unit, leading to two types of infinite π-stacking pathways; one such strand is illustrated in Fig. 2. Stacking arrangements of this type probably contribute towards charge-transport in conjugated polyrotaxanes, and suggest an explanation for the semiconductivity of these insulated molecular wires.5
![Synthesis of the [2]rotaxane. Reagents and conditions:
α-cyclodextrin, H2O, NaOH, Na2CO3, Pd(OAc)2, 50 °C, then dilute HCl aq.](/image/article/2004/CC/b311762f/b311762f-s1.gif) | ||
| Scheme 1 Synthesis of the [2]rotaxane. Reagents and conditions: α-cyclodextrin, H2O, NaOH, Na2CO3, Pd(OAc)2, 50 °C, then dilute HCl aq. | ||
 | ||
| Fig. 1 View of the two rotaxane molecules in the asymmetric unit of 3⊂α-CD (H atoms and water molecules omitted for clarity). | ||
 | ||
| Fig. 2 Two orthogonal views of a strand of π–π stacked rotaxane molecules in the crystal structure of 3⊂α-CD. | ||
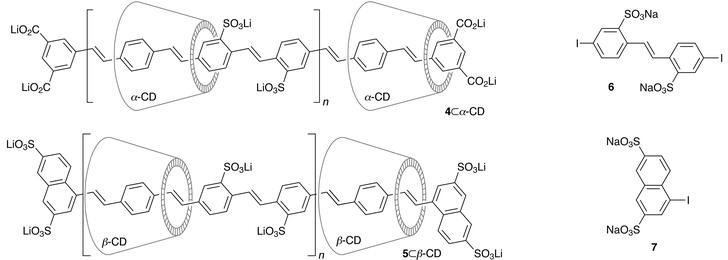
PPV-polyrotaxanes 4⊂α-CD and 5⊂β-CD were prepared under similar conditions, by coupling 1 with diiodostilbene 6 and chain-terminators 2 and 7, in the presence of α- and β-cyclodextrin respectively.† A polymerisation stoichiometry of [6]/[2]
=
[6]/[7]
= 0.20 was used to give an expected number-average degree of polymerisation of ![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) = 10, which compares well with the experimental values from analytical ultracentrifugation of
= 10, which compares well with the experimental values from analytical ultracentrifugation of ![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) = 6.8 for 4⊂α-CD and
= 6.8 for 4⊂α-CD and ![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) = 12.3 for 5⊂β-CD.7†1H NMR analysis shows that the average number of cyclodextrins per unsubstituted para-phenylene is 0.7 ± 0.1 for 4⊂α-CD and 1.0 ± 0.1 for 5⊂β-CD. Extensive dialysis with a 5000 nominal molecular weight cut-off membrane did not change the amount of threaded cyclodextrin, demonstrating that the ends of the chain are effectively capped.
= 12.3 for 5⊂β-CD.7†1H NMR analysis shows that the average number of cyclodextrins per unsubstituted para-phenylene is 0.7 ± 0.1 for 4⊂α-CD and 1.0 ± 0.1 for 5⊂β-CD. Extensive dialysis with a 5000 nominal molecular weight cut-off membrane did not change the amount of threaded cyclodextrin, demonstrating that the ends of the chain are effectively capped.
The absorption and emission spectra of the rotaxanes 3⊂α-CD, 4⊂α-CD and 5⊂β-CD are compared with those of the uninsulated dumbbells in Table 1. As previously observed in other polyrotaxanes of this type,5–8 the presence of the cyclodextrin has little effect on the absorption spectra, but causes a marked blue shift in the emission of 4⊂α-CD and 5⊂β-CD. In every case, the threaded cyclodextrin increases fluorescence efficiencies. All the polymers are much less emissive than 3 and 3⊂α-CD; this seems to be a general feature of PPV-polyelectolytes.11 Work is in progress towards the synthesis of non-polar versions of these insulated molecular wires.
| Compound | λ max abs/nm | λ max em/nm | Φ f |
|---|---|---|---|
| a All spectra in pH 9 aqueous buffer (5 mM LiOH, 5 mM Li2CO3); fluorescence quantum yields, Φf, are accurate to ±10% and were determined relative to quinine sulfate in 0.5 M H2SO4 (Φf 0.546). | |||
| 3⊂α-CD | 360 | 420 | 1.0 |
| 3 | 356 | 418 | 0.80 |
| 4⊂α-CD | 436 | 484 | 0.14 |
| 4 | 433 | 519 | 0.05 |
| 5⊂β-CD | 438 | 476 | 0.08 |
| 5 | 437 | 522 | 0.03 |
This work was supported by the EPSRC and the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Jonathan M. Elkins, David J. Watkin and Andrew R. Cowley for crystallographic advice.
Notes and references
- R. J. O. M. Hoofman, M. P. de Haas, L. D. A. Siebbles and J. M. Warman, Nature, 1998, 392, 54 CrossRef CAS; W. B. Davis, W. A. Svec, M. A. Ratner and M. R. Wasielewski, Nature, 1998, 396, 60 CrossRef CAS; H. D. Sikes, J. F. Smalley, S. P. Dudek, A. R. Cook, M. D. Newton, C. E. D. Chidsey and S. W. Feldberg, Science, 2001, 291, 1519 CrossRef CAS.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. MacKay, R. H. Friend, P. L. Burn and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS; A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed., 1998, 37, 403 CrossRef CAS.
- L. Chen, D. W. McBranch, H.-L. Wang, R. Helgeson, F. Wudl and D. G. Whitten, Proc. Natl. Acad. Sci. USA, 1999, 96, 12287 CrossRef CAS.
- F. Hide, M. A. Díaz-García, B. J. Schwartz and A. J. Heeger, Acc. Chem. Res., 1997, 30, 430 CrossRef CAS.
- F. Cacialli, J. S. Wilson, J. J. Michels, C. Daniel, C. Silva, R. H. Friend, N. Severin, P. Samorì, J. P. Rabe, M. J. O'Connell, P. N. Taylor and H. L. Anderson, Nat. Mater., 2002, 1, 160 Search PubMed.
- P. N. Taylor, M. J. O'Connell, L. A. McNeill, M. J. Hall, R. T. Aplin and H. L. Anderson, Angew. Chem., Int. Ed., 2000, 39, 3456 CrossRef CAS.
- J. J. Michels, M. J. O'Connell, P. N. Taylor, J. S. Wilson, F. Cacialli and H. L. Anderson, Chem. Eur. J., 2003, 9, in press CrossRef CAS.
- C. A. Stanier, S. J. Alderman and T. D. W. Claridge and H. L. Anderson, Angew. Chem., Int. Ed., 2002, 41, 1769 CrossRef; C. A. Stanier, M. J. O'Connell, W. Clegg and H. L. Anderson, Chem. Commun., 2001, 493 RSC.
- J. E. H. Buston, F. Marken and H. L. Anderson, Chem. Commun., 2001, 1046 RSC; J. E. H. Buston, J. R. Young and H. L. Anderson, Chem. Commun., 2000, 905 RSC.
- Y. Uozumi and Y. Nakai, Org. Lett., 2002, 4, 2997 CrossRef CAS; M. Murata, S. Watanabe and Y. Masuda, Tetrahedron Lett., 1999, 40, 2585 CrossRef CAS; D. Badone, M. Baroni, R. Cardamone, A. Ielmini and U. Guzzi, J. Org. Chem., 1997, 62, 7170 CrossRef CAS.
- M. W. Wagaman and R. H. Grubbs, Macromolecules, 1997, 30, 3978 CrossRef CAS; X. Chen and F. Wudl, Polym. Prepr. (ACS, Div. Pol. Chem.), 2002, 43, 19 Search PubMed.
- J. Foadi, M. M. Woolfson, E. J. Dodson, K. S. Wilson, Y. Jia-xing and Z. Chao-de, Acta. Crystallogr., Sect. D, 2000, 56, 1137 CrossRef.
- M. C. Burla, B. Carrozzini, G. L. Cascarano, C. Giacovazzo and G. Polidori, Z. Kristallogr., 2002, 217, 629 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: details of analytical ultracentrifuge measurements; experimental synthetic procedures. See http://www.rsc.org/suppdata/cc/b3/b311762f/ |
| ‡ Crystal data for 3⊂α-CD: C62H78O38·12H2O, M = 1647.5, triclinic, space group P1, a = 13.8627(3), b = 17.1623(3), c = 20.0027(4) Å, α = 65.1372(7), β = 70.6427(7), γ = 66.0815(7)°; V = 3872.01(13) Å3, Z = 2, T = 150 K, λ = 0.71073 Å, μ(Mo-Kα) = 0.124 mm−1, 17278 independent reflections, 11696 with I > 2.5σ(I); R1 = 0.0931, Rw2 = 0.1091. Data were collected on an Enraf Nonius Kappa CCD diffractometer, and solved and refinement using programs ACORN,12 SIR200213 and CRYSTALS (SIR92 and SIR97 were unable to solve this structure). The structure was refined on F. H atoms were placed geometrically on carbon; some H atoms were tentatively located on oxygen from the electron density map; H coordinates were not refined. The asymmetric unit contains two molecules of 3⊂α-CD and ca. 24 molecules of water, disordered over 36 sites. The crystals are delicate and easily lose water. In one of the rotaxane molecules, the isophthalic acid unit further from the cyclodextrin is disordered over two orientations (weightings 53 : 47); only the higher occupancy coordinates are plotted in Fig. 1. CCDC 220680. See http://www.rsc.org/suppdata/cc/b3/b311762f/ for crystallographic data in CIF or other electronic format. |
| § π–π Stacking, CH⋯π and OH⋯O interactions in this structure are characterised by distances of C⋯C 3.45–3.55 Å, H⋯C 2.65–2.85 Å, and O⋯O 2.55–2.95 Å respectively. |
| This journal is © The Royal Society of Chemistry 2004 |